Preparation method for tert-butyl acetate
A technology of tert-butyl acetate and acetic acid, applied in the field of preparation of tert-butyl acetate, can solve problems such as low mechanical strength, and achieve the effects of reducing costs, suppressing side reactions, and reducing the amount of water used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
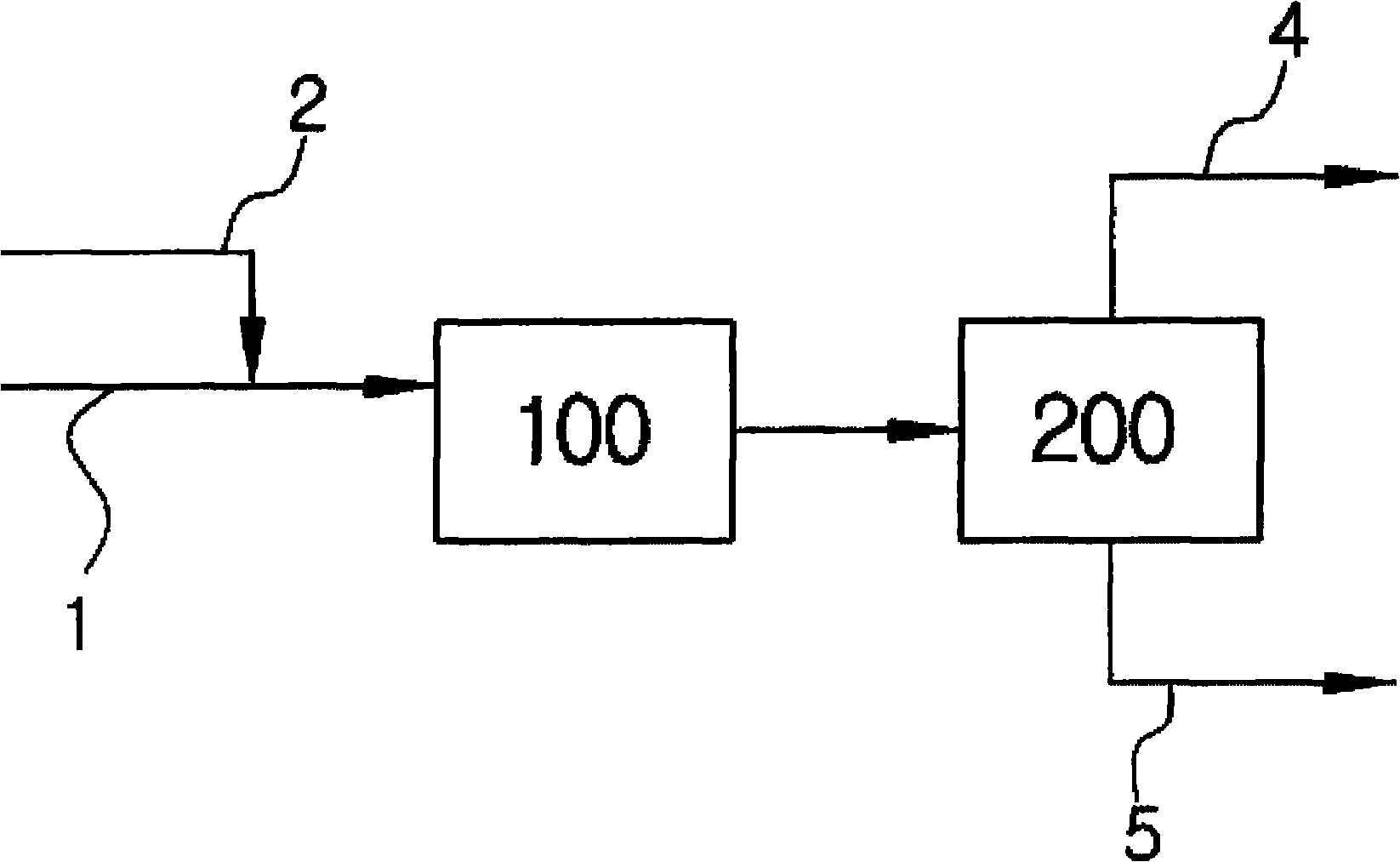
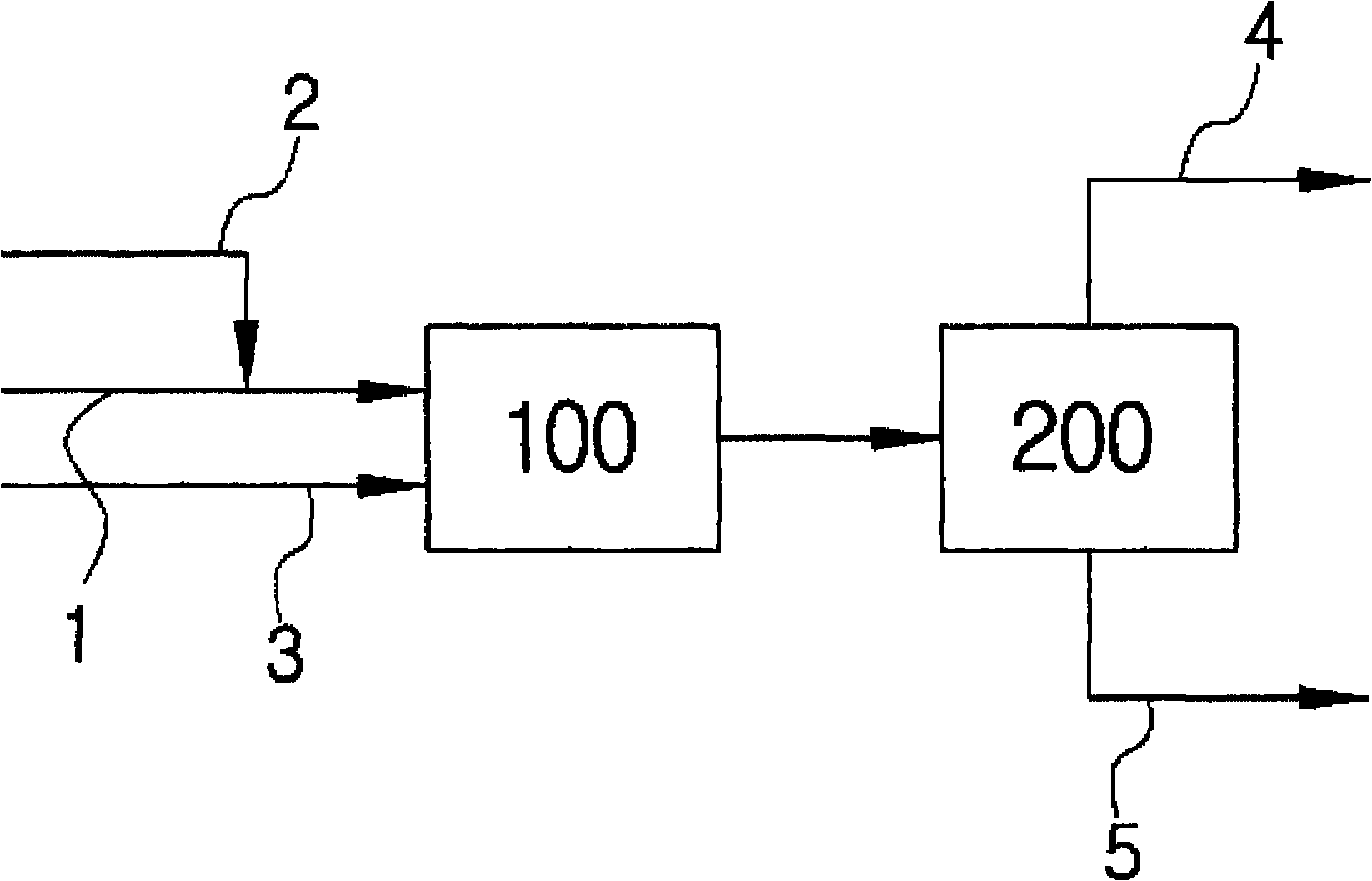
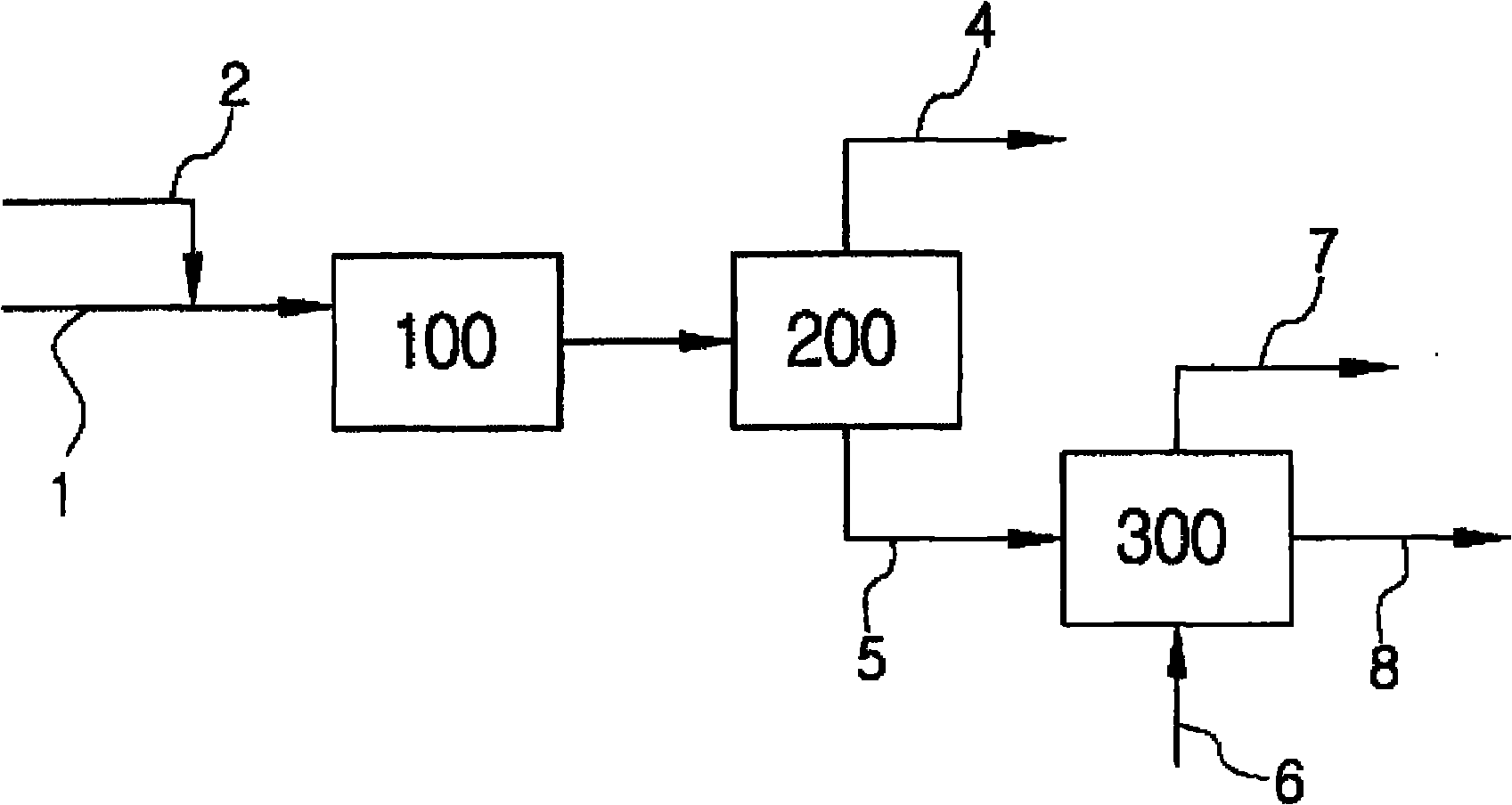
[0041] The tubular reactor was filled with 10 ml of Amberyst-15 catalyst, and the olefin mixture with four carbon atoms and acetic acid were injected through the HPLC pump at a volume space velocity of 10 / h. The volume percentage of isobutene in the olefin is 50%, the other components are n-butane, isobutane, 1-butene and 2-butene, and the used olefin mixture is the mixture from which butadiene has been extracted. The ratio of isobutene to acetic acid in the olefin is 1:4. A water bath (Bath) was used to maintain the reaction temperature at 35° C., and a Back Pressure Regulator (Back Pressure Regulator) was installed in the reservoir (Reservior) at the rear end of the reactor to adjust the pressure to 10 atmospheres. The storage tank is cooled with dry ice so that the reacted olefins with four carbon atoms and hydrocarbons can be condensed without loss. The reaction product was analyzed by gas chromatography (GC).
Embodiment 2
[0043] Under the same conditions as in Example 1, a porous surface-modified zeolite was used as the catalyst, and the zeolite was obtained by introducing 10 mol% of sulfonic acid groups into mordenite.
Embodiment 3
[0045] Under the same conditions as in Example 1, water of 5 mol % of isobutene content was mixed in acetic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com