Medicinal sustained-release preparation for treating osteomyelitis and preparation method and application thereof
A slow-release preparation and osteomyelitis technology, which is applied in the direction of anti-inflammatory agents, drug combinations, drug delivery, etc., to achieve the effects of convenient use, significant therapeutic effect, and simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A drug sustained-release preparation for preventing and treating osteomyelitis, which is made of the following raw materials in the mass ratio w / w:
[0058] Raw material mass ratio w / w
[0059] Racemic polylactic acid The molecular weight of racemic polylactic acid is 40,000-120,000, 66.67%-90%
[0060] Levofloxacin 10%-33.33%
[0061] A kind of preparation method of the drug sustained release preparation of preventing and treating osteomyelitis, its steps are:
[0062] A. Cut the racemic polylactic acid material into fragments of equal size (such as 5×5mm 2 ), add the corresponding mass of levofloxacin powder, place it in an organic solvent with 4 to 5 times the w / v of the total mass of the blended material and stir at a constant speed until it is completely dissolved. Fast volatilization, the powder blend of racemic polylactic acid and levofloxacin is attached to the container wall and dried;
[0063] B. Heat the blend of racemic polylactic acid and levofloxacin i...
experiment example 1
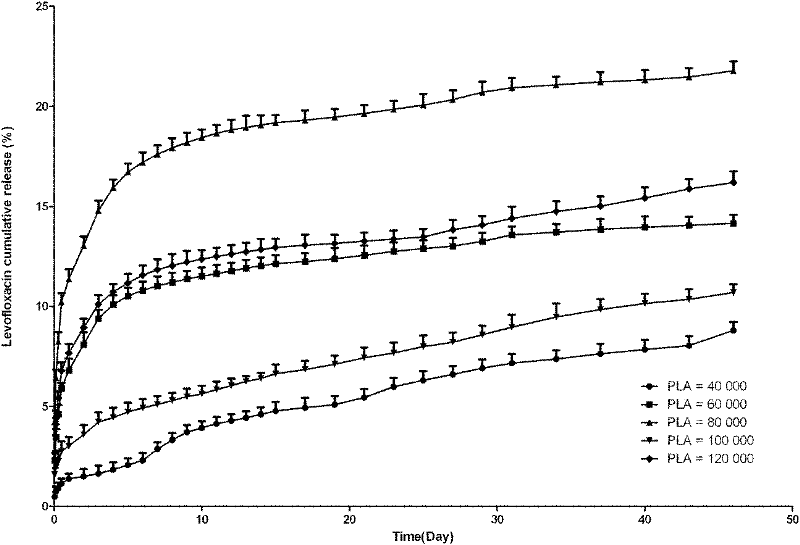
[0073] Experimental example 1: In vitro drug release experiment of racemic polylactic acid and levofloxacin blended sustained-release tablets 1
[0074] Sampling: set the mass of racemic polylactic acid and levofloxacin blended slow-release tablets to be 100 mg, get the racemic polylactic acid with molecular weights of 4, 6, 8, 10, and 120,000 respectively, and the ratio of racemic polylactic acid and levofloxacin is 8: 3 pieces of 2w / w tablets were placed in test tubes respectively, 10ml of 0.1M phosphate buffer (pH 7.4) was added to each, 37°C, rotating speed 60r / min to simulate in vivo movement. The phosphate buffer was changed at 1, 2, 4, 8, and 12 hours after the experiment started; the phosphate buffer was changed every day from the 1st to 15th day of the experiment, and every 2 days from the 16th to 31st day; On the 32nd day, the phosphate buffer was changed every 3 days until 46 days after release. After the liquid was taken out and centrifuged, the supernatant liquid...
experiment example 2
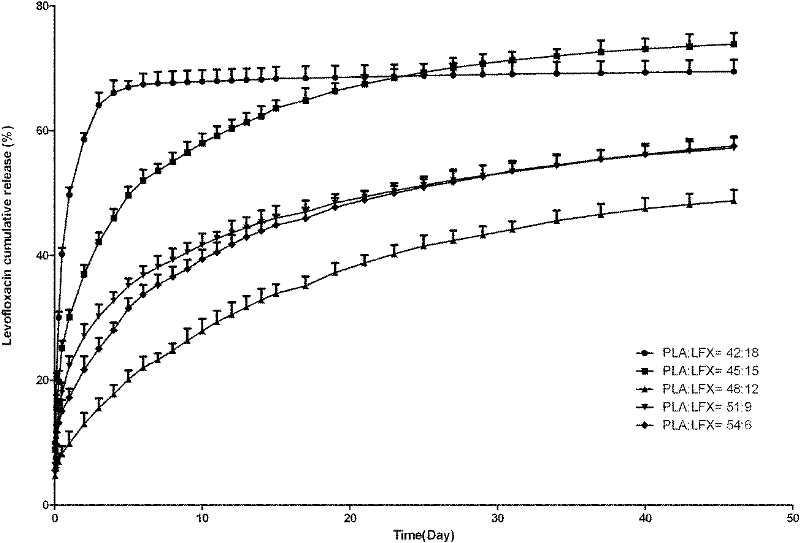
[0079] Experimental Example 2: In vitro drug release experiment of racemic polylactic acid and levofloxacin blended sustained-release tablets 2
[0080] Sampling: Set the mass of racemic polylactic acid and levofloxacin blended sustained-release tablets to 60 mg, and the ratios of racemic polylactic acid and levofloxacin are 54:6, 51:9, 48:12, 45:15 and 42:18w / w respectively Each of the 3 tablets was placed in a test tube, and 10ml of 0.1M phosphate buffer (pH 7.4) was added to each of them, at 37°C, at a speed of 60r / min to simulate the movement in the body. The phosphate buffer was changed at 1, 2, 4, 8, and 12 hours after the experiment started; the phosphate buffer was changed every day from the 1st to 15th day of the experiment, and every 2 days from the 16th to 31st day; On the 32nd day, the phosphate buffer was changed every 3 days until 46 days after release. After the liquid was taken out and centrifuged, the supernatant was taken into a test tube and packaged, and s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com