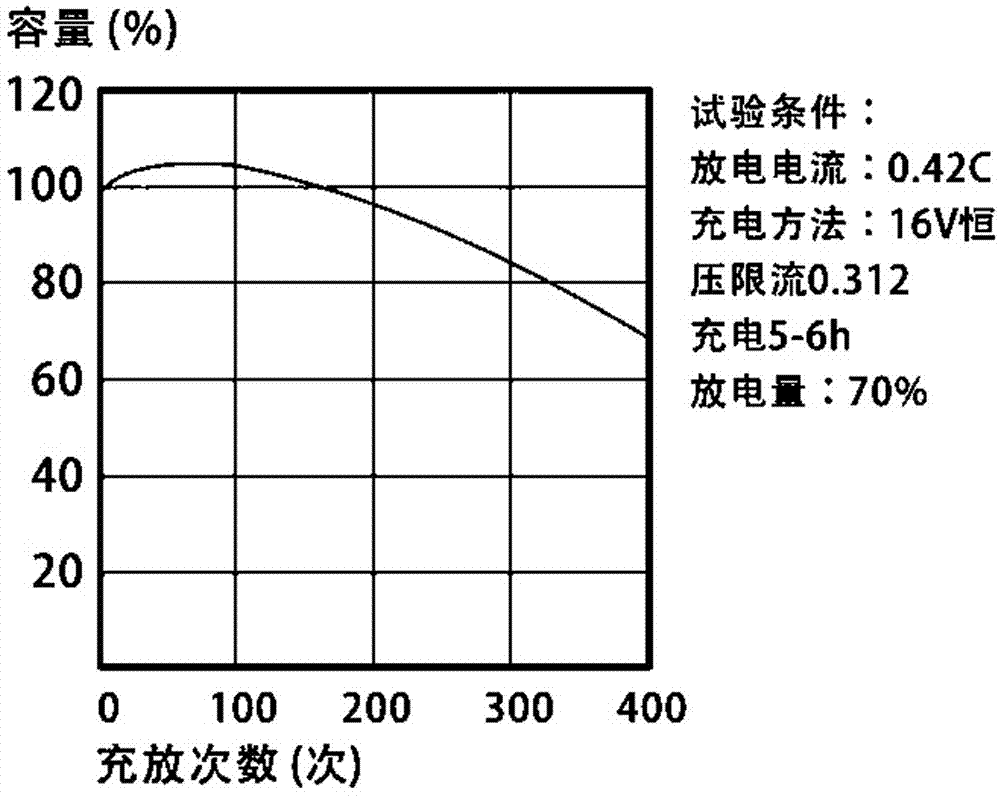
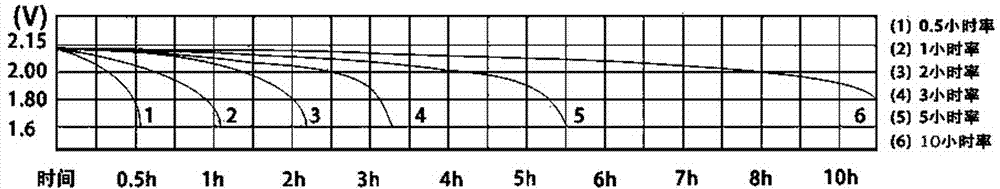
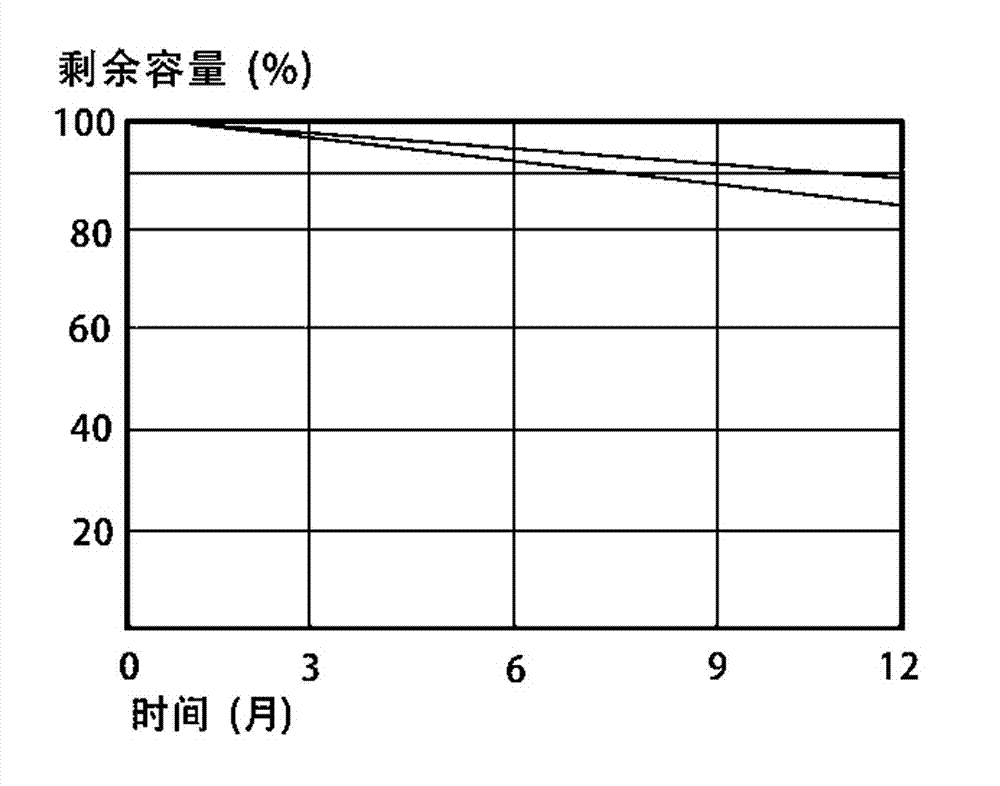
Novel silicate electrolyte storage battery
An electrolyte and silicate technology, applied in lead-acid batteries and other directions, can solve the problems of irreversible damage to personnel and the environment, difficult recovery of electrolyte, poor battery performance, etc., to achieve no charge and discharge memory effect, small self-discharge, and improved The effect of the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Preparation of electrolyte: Add deionized water to 10% silica and stir at high speed to disperse; after the dispersion is uniform, add 40% sulfuric acid, stir for reaction, then add 1% ammonium phosphate, 2% ammonium persulfate and potassium sulfate 1.5%, mixing and stirring reaction; after the reaction is completed, add 1.5% polyacrylamide, 1% polyvinyl alcohol, 3% sodium carboxymethyl cellulose and 4% silicone oil, stir in a sealed environment at normal temperature and pressure, and catalyze the reaction for 24 hours, then After 8000 Gauss magnetization treatment for 10 seconds, the electrolyte was prepared;
[0042] (2) The electrolyte is poured into the battery and packaged.
Embodiment 2
[0044] (1) Preparation of electrolyte: Add deionized water to 10% silica and stir at high speed to disperse; after the dispersion is uniform, add 45% sulfuric acid, stir for reaction, then add 1% ammonium phosphate, 2% ammonium persulfate and potassium sulfate 1%, mixed and stirred for reaction; after the reaction is completed, add 1% polyacrylamide, 1% glycerin, 2% sodium carboxymethylcellulose and 4% silane, stir in a sealed environment at normal temperature and pressure, and catalyze the reaction for 12 hours, then After 10,000 Gauss magnetization treatment for 15 seconds, the electrolyte was prepared;
[0045] (2) The electrolyte is poured into the battery and packaged.
Embodiment 3
[0047] (1) Preparation of electrolyte: add deionized water to 15% silica and stir at high speed to disperse; after the dispersion is uniform, add 30% sulfuric acid, stir for reaction, then add 1.3% ammonium phosphate, 3% ammonium persulfate and potassium sulfate 1%, mixed and stirred for reaction; after the reaction is completed, add polyacrylamide 1.5%, glycerin 2%, sodium carboxymethylcellulose 2% and silicone oil 3%, stir in a sealed environment at normal temperature and pressure, and catalyze the reaction for 12 hours, then After 8000 Gauss magnetization treatment for 15 seconds, the electrolyte was prepared;
[0048] (2) The electrolyte is poured into the battery and packaged.
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com