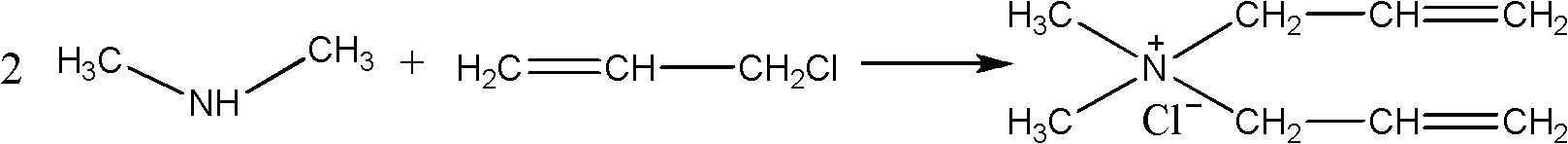Method for synthesizing dimethyl diallyl ammonium chloride cationic monomer
A technology of dimethyl diallyl ammonium chloride and cationic monomers, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of hydroxyl compounds, etc. It can solve the problem of not doing too much research on feeding methods and increasing the production cost of the process , There are problems such as temperature limitations, to achieve the effect of increased stability, reduced loss, and reduced loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
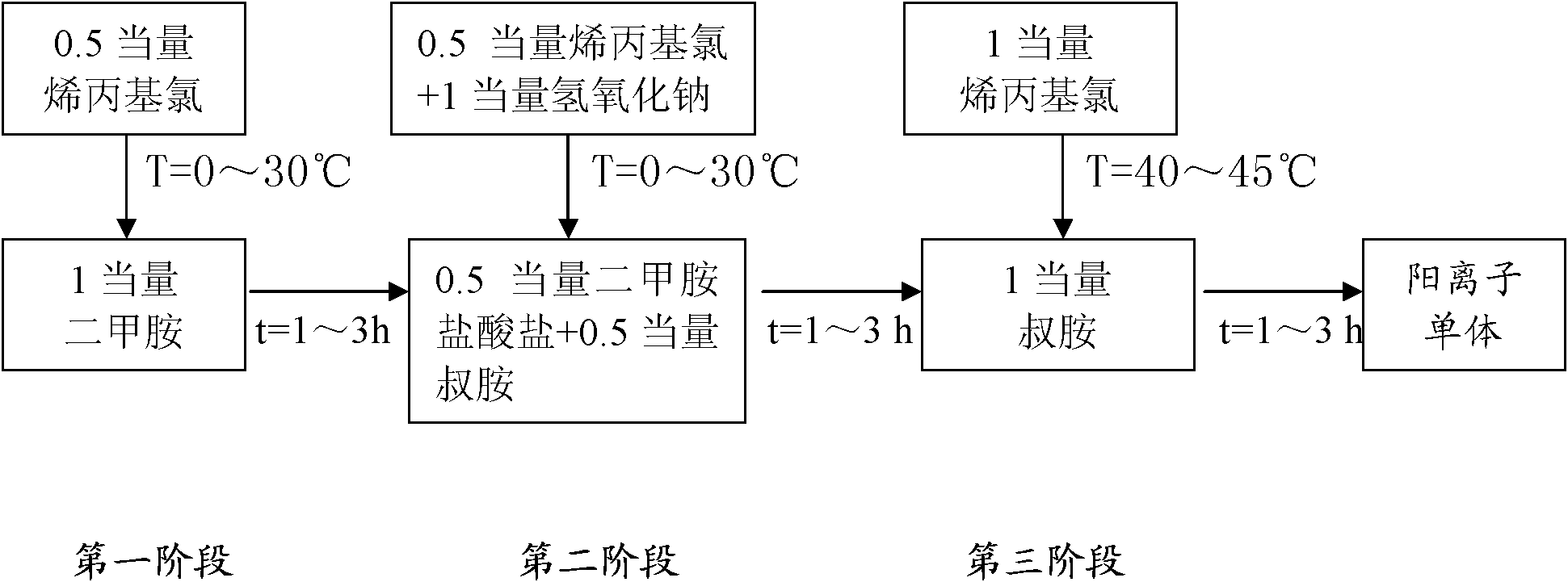
[0063] (1) Take a 40% aqueous solution containing 0.8mol dimethylamine in a mass fraction, place it in a 500ml four-necked round-bottomed flask, add 0.4mol allyl chloride dropwise at a temperature of 0°C, drop it in 1 hour, and carry out the first Phase reaction, the reaction temperature is 0 °C, and the gas chromatography is turned on to monitor the reaction process. After 3 hours of reaction, the content of allyl chloride in the system is monitored to be zero, and the next step is started;
[0064] (2) Alternately add 0.4mol allyl chloride and 0.8mol sodium hydroxide dropwise at a temperature of 10°C. After 1h, the second stage reaction is carried out. The reaction temperature is 10°C. After 3 hours of reaction, the substances in the system are basically When tertiary amine starts to carry out next step operation;
[0065] (3) Continue to add 0.8mol allyl chloride dropwise at 25° C. After 1 hour of dripping, carry out the third-stage reaction. The reaction time is 7 hours, a...
Embodiment 2
[0076] (1) Take a 40% aqueous solution containing 0.8mol dimethylamine in a mass fraction, place it in a 500ml four-necked round bottom flask, add 0.4mol allyl chloride dropwise at a temperature of 10°C, drop it in 2 hours, and carry out the first Phase reaction, the reaction temperature is 10°C, and the gas chromatography is turned on to monitor the reaction process at the same time. After 2 hours of reaction, the content of allyl chloride in the system is monitored to be zero, and the next step is started;
[0077] (2) Add 0.4mol allyl chloride and 0.8mol sodium hydroxide dropwise at the same time at a temperature of 30°C. After 2 hours, the second stage reaction is carried out. The reaction temperature is 30°C. After 2 hours of reaction, the substances in the system are basically When tertiary amine starts to carry out next step operation;
[0078] (3) Continue to add 0.8mol allyl chloride dropwise at 25° C., drop it over 2 hours, and carry out the third stage reaction. The...
Embodiment 3
[0083] (1) Take a 40% aqueous solution containing 0.8mol dimethylamine in a mass fraction, place it in a 500ml four-neck round bottom flask, add 0.4mol allyl chloride dropwise at a temperature of 10°C, drop it in 3 hours, and carry out the first Phase reaction, the reaction temperature is 10°C, and the gas chromatograph is turned on to monitor the reaction process at the same time. After 1 hour of reaction, the content of allyl chloride in the system is monitored to be zero, and the next step is started;
[0084] (2) Add 0.4 mol allyl chloride and 0.8 mol sodium hydroxide alternately or simultaneously at 20°C, drop it in 3 hours, and proceed to the second stage reaction, the reaction temperature is 20°C, and the system is monitored after 1 hour of reaction When the substance in the middle is basically a tertiary amine, start to carry out the next step;
[0085] (3) Continue to add 0.8mol allyl chloride dropwise at 25° C., drop it over 2 hours, and carry out the third-stage rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com