Production process for preparing high-purity ApoA-I (Apolipoprotein A-I) from precipitates of plasma fraction IV
A technology of plasma component four and pH value, which is applied in the field of production technology for preparing high-purity apolipoprotein ApoA-I, can solve the problems of loss of ApoA-I, unfavorable safe production, small preparation amount, etc., and achieves safe and convenient operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
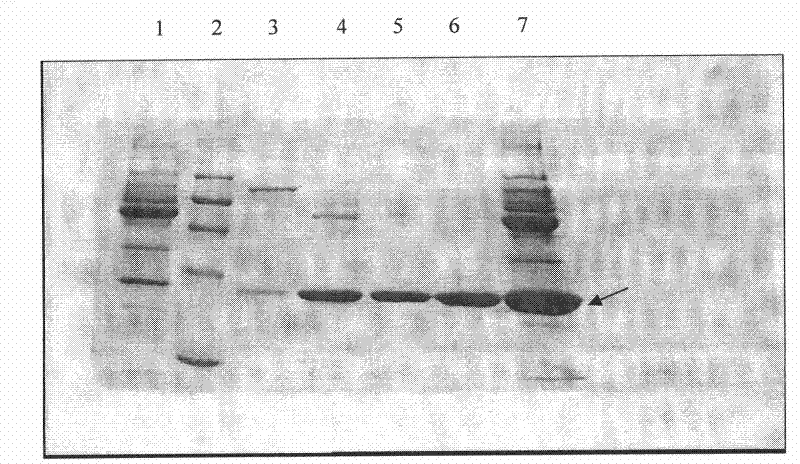
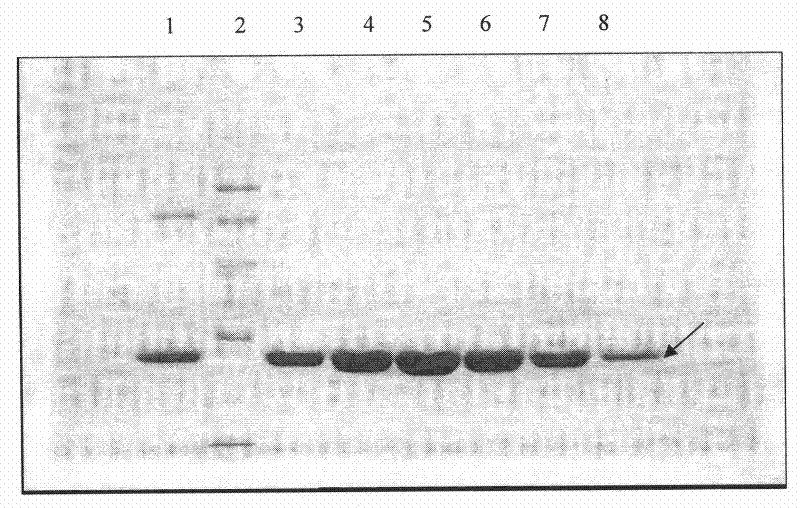
Image
Examples
Embodiment 1
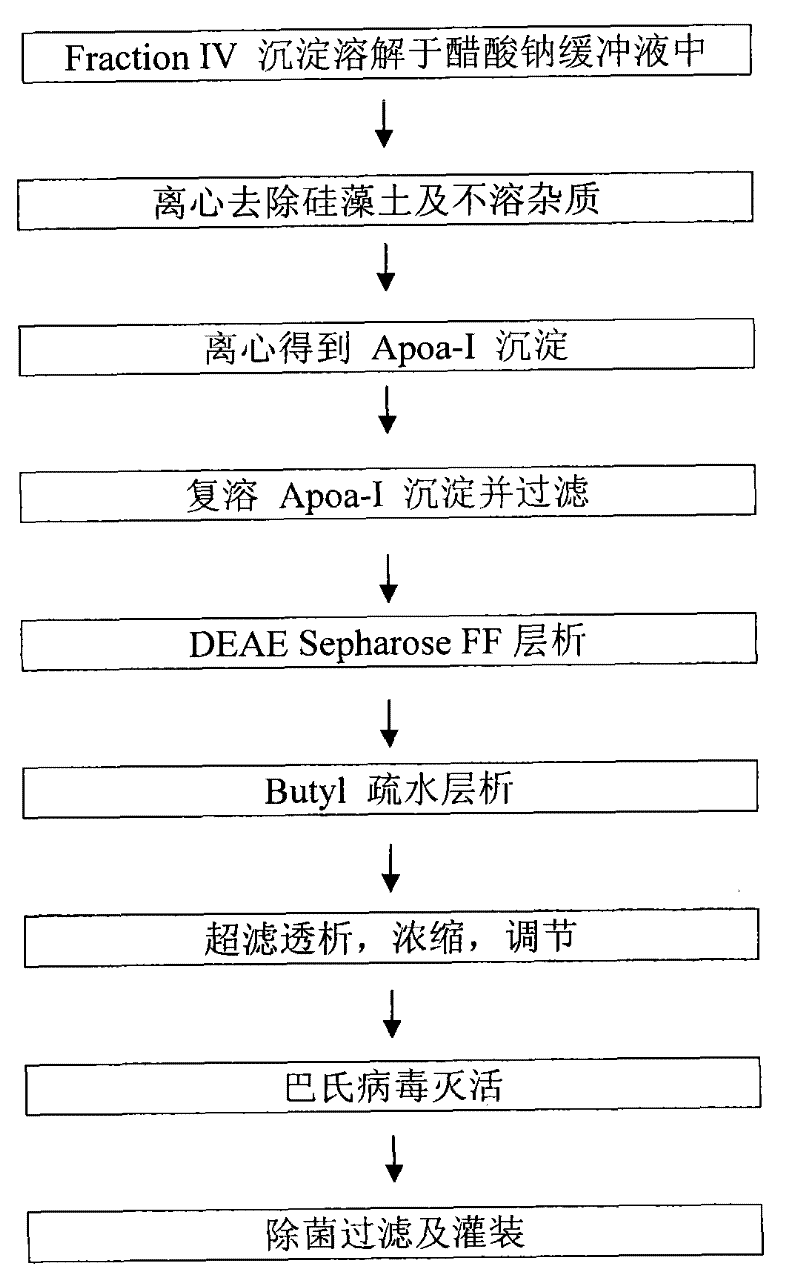
[0035] Embodiment 1: ApoA-I sample preparation
[0036] (1) Plasma component four precipitation dissolution and pretreatment
[0037] Weigh 5 kg (wet weight) of component 4 precipitate, dissolve in 45 kg of sodium acetate buffer solution (control temperature 0-8°C), adjust the pH value to about 9.00, stir well to dissolve it, and then centrifuge it with Beckmann machine (7000rpm) to remove diatomaceous earth and insoluble matter, and collect the centrifuged supernatant.
[0038] (2) centrifugation to obtain ApoA-I precipitate
[0039] Sodium chloride is added to the supernatant to make the concentration reach about 2wt%, and then the pH value is adjusted to 6.0-6.5, and the temperature is lowered to -1-1°C to allow ApoA-I to coagulate and precipitate. Centrifuge at high speed, collect about 500 g of ApoA-I precipitate, and discard the supernatant.
[0040] (3) Redissolve ApoA-I precipitation
[0041] Fully dissolve 500 g of ApoA-I precipitate in 5 kg of 2 wt % sodium chlor...
Embodiment 2
[0051] ApoA-I sample preparation
[0052] (1) Plasma component four precipitation dissolution and pretreatment
[0053] Weigh 3 kg (wet weight) of the precipitate of component four, dissolve it in sodium acetate buffer (control temperature: 0-8°C), adjust the pH value to 8.00, stir well to dissolve it, and then centrifuge at 7000rpm to remove diatomaceous earth and Insoluble matter was collected by centrifugation supernatant.
[0054] (2) centrifugation to obtain ApoA-I precipitate
[0055] Sodium chloride is added to the supernatant to make the concentration reach 1wt%, then the pH value is adjusted to 6.0-6.5, and the temperature is lowered to -1-1° C. to allow ApoA-I to coagulate and precipitate. Centrifuge at high speed, collect about 300 g of ApoA-I precipitate, and discard the supernatant.
[0056] (3) Redissolve ApoA-I precipitation
[0057] Fully dissolve 300 g of the ApoA-I precipitate in 1 wt% sodium chloride solution at 0° C., and then filter with a 0.45 μm filt...
Embodiment 3
[0062] ApoA-I sample preparation
[0063] (1) Plasma component four precipitation dissolution and pretreatment
[0064] Weigh 6 kg (wet weight) of the precipitate of component four, dissolve it in sodium acetate buffer solution (control temperature is 0-8°C), adjust the pH value to 10.00, stir well to dissolve it, and then remove diatomaceous earth by centrifugation at 7000rpm and insoluble matter, and the centrifuged supernatant was collected.
[0065] (2) centrifugation to obtain ApoA-I precipitate
[0066] Sodium chloride is added to the supernatant to make the concentration reach 3wt%, and then the pH value is adjusted to 6.0-6.5, and the temperature is lowered to -1-1° C. to allow ApoA-I to coagulate and precipitate. Centrifuge at high speed, collect about 600 g of ApoA-I precipitate, and discard the supernatant.
[0067] (3) Redissolve ApoA-I precipitation
[0068] Fully dissolve 600 g of ApoA-I precipitate in 3 wt% sodium chloride solution at about 10° C., and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com