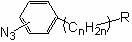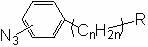Photosensitive coupling molecule and synthesis method and application thereof
A molecular and coupling technology, applied in the field of chemistry and materials, can solve the problems of the limitation of the total amount of modification, the restriction of the effect of material modification, and the low coupling efficiency of macromolecular end groups, so as to achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of compound (1), structural formula is as follows,
[0026]
[0027] Add 3.5 mL of 2 mol / L sodium nitrite solution into a 100 mL flask, then add 10 mL of 2 mol / L hydrochloric acid, take 430 mg (~ 2.8 mmol) p-aminophenylacetic acid, add it to the solution, and place in a low-temperature ice bath, Protect from light for 30 min; then slowly add 3.2 mL of 1 mol / L sodium azide aqueous solution, and complete the addition in about 1.5 h. The mixture was transferred to a sand core funnel for filtration, the solid phase was transferred and dissolved in ethyl acetate, 10 g of anhydrous magnesium sulfate powder was added, stirred and dried for 24 h, and then filtered with a dry sand core funnel, and the filtrate was rotary evaporated to remove the solvent.
[0028] Take 260 mg of the above product (~ 1.5 mmol) into a 100 mL flask filled with dry argon, add 6 mL of dehydrated dichloromethane, and add 350 mg (~ 3.0 mmol) of For NHS, take 7.5 mg of D...
Embodiment 2
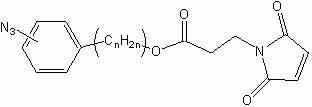
[0029] Embodiment 2: the preparation of compound (2), structural formula is as follows,
[0030]
[0031] Add 3.5 mL of 2 mol / L sodium nitrite solution to a 100 mL flask, then add 10 mL of 2 mol / L hydrochloric acid, take 460 mg (~ 2.8 mmol) of m-aminophenylpropionic acid, add it to the solution, and place in a low-temperature ice bath , and reacted in the dark for 30 min; then slowly added 3.2 mL of 1 mol / L sodium azide aqueous solution, and the addition was completed in about 1.5 h. The mixture was transferred to a sand core funnel for filtration, the solid phase was transferred and dissolved in ethyl acetate, 10 g of anhydrous magnesium sulfate powder was added, stirred and dried for 24 h, and then filtered with a dry sand core funnel, and the filtrate was rotary evaporated to remove the solvent.
[0032] Take 285 mg of the above product (~ 1.5 mmol) into a 100 mL flask filled with dry argon, add 6 mL of dehydrated dichloromethane, and add 350 mg (~ 3.0 mmol) of For NHS,...
Embodiment 3
[0033] Embodiment 3: the preparation of compound (3), structural formula is as follows,
[0034]
[0035] Add 3.5 mL of 2 mol / L sodium nitrite solution into a 100 mL flask, then add 10 mL of 2 mol / L hydrochloric acid, take 495 mg (~ 2.8 mmol) p-aminobenzenebutyric acid, add it to the solution, and place in a low-temperature ice bath, Protect from light for 30 min; then slowly add 3.2 mL of 1 mol / L sodium azide aqueous solution, and complete the addition in about 1.5 h. The mixture was transferred to a sand core funnel for filtration, the solid phase was transferred and dissolved in ethyl acetate, 10 g of anhydrous magnesium sulfate powder was added, stirred and dried for 24 h, and then filtered with a dry sand core funnel, and the filtrate was rotary evaporated to remove the solvent.
[0036]Take 300 mg of the above product (~ 1.5 mmol) into a 100 mL flask filled with dry argon, add 6 mL of dehydrated dichloromethane, and add 350 mg (~ 3.0 mmol) of For NHS, take 7.5 mg of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com