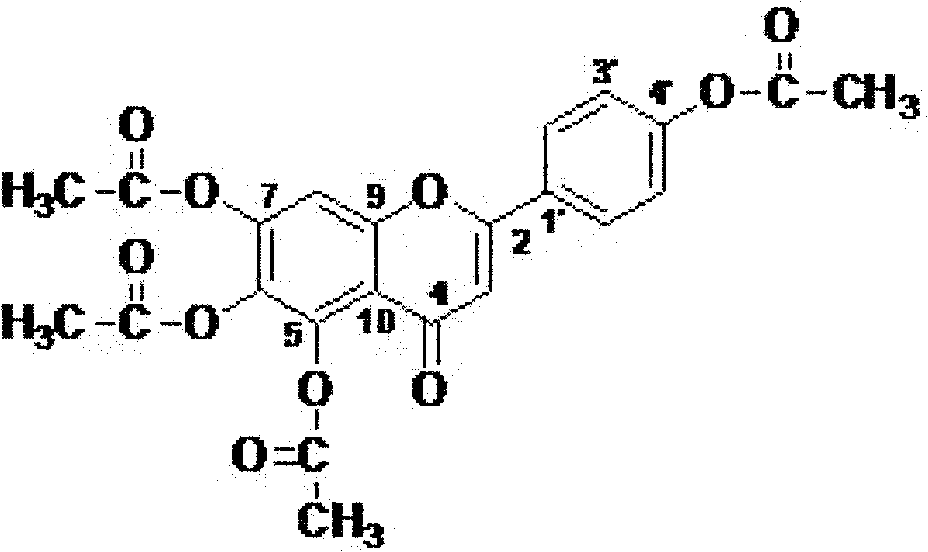
Use of 5,6,7,4'-tetra-acetoxyl flavone for preparing medicine for preventing and curing cardiovascular and cerebrovascular diseases
A technology for tetraacetoxyflavones and cardiovascular and cerebrovascular diseases, which is applied in the field of medicine and can solve the problems of easy oxidation and deterioration of phenolic hydroxyl groups and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Synthesis of 5,6,7,4'-tetraacetoxyflavone
[0016] Put 10.0g of 5,6,7,4'-tetrahydroxyflavone and 75ml of acetic anhydride in a 300ml Erlenmeyer flask, add 1 drop of concentrated hydrochloric acid (about 0.1ml), stir magnetically at room temperature for 1 hour, and detect the raw material point by TLC Disappeared, filtered and washed to neutral, and the filtrate was dried to obtain 5,6,7,4'-tetraacetoxyflavone. The yield is 92%, and the purity is 98% as detected by HPLC.
[0017] UV max (MeOH)(Abs.): 252(0.395), 278(0.585), 388(0.156)nm.IR KBr max : 3441, 1778, 11651, 1456, 1372, 1216, 1199, 1080, 1016, 840cm -1 .FAB-MS: m / z 455[M+H] + , 413 [M+H-COCH 2 ] + , 370[M+H-COCH 2 -COCH 2 ] + , 328 [M+H-COCH 2 -COCH 2 -COCH 2 ] + , calcd for C 23 h 19 o 10 , 455.0978. Molecular formula is C 23 h 18 o 10 , with a molecular weight of 454. 1 H (500MHz, DMSO-d 6 ) and 13 C NMR (125MHz, DMSO-d 6 ): See Table 1
[0018] Table 1.5,6,7,4′-Tetrahydro...
Embodiment 2
[0021] Example 2: Acute toxicity test of 5,6,7,4'-tetraacetoxyflavone on mice
[0022] Tested drug: 5,6,7,4'-tetraacetoxyflavone, provided by Kunming Pharmaceutical Group Co., Ltd. Drug Research Institute, batch number 20070312, grinded evenly with 0.5% sodium carboxymethylcellulose before use , dubbed into a suspension with a concentration of 0.1g / ml.
[0023] Experimental animals: ICR mice 18-22g, 20, male and female, provided by the Animal Laboratory of Kunming Pharmaceutical Group Co., Ltd., production license number SCXK (Dian) 2005-0006, use license number SYXK (Dian) 2005 -0006.
[0024] Test method and results: Select healthy ICR mice, weighing 20±2g, 20, half male and half male, orally gavage the mice with 5,6,7,4′-tetraacetoxyflavone with a concentration of 0.1g / ml Suspension 0.8ml / 20g body weight, continuous observation for 7 days, the mice were normal and agile, and did not cause death or abnormal reaction.
Embodiment 3
[0025] Embodiment 3: Chronic toxicity test experiment of 5,6,7,4'-tetraacetoxyflavone to rats
[0026] Tested drug: 5,6,7,4'-tetraacetoxyflavone, provided by Kunming Pharmaceutical Group Co., Ltd. Drug Research Institute, batch number 20070312, grinded evenly with 0.5% sodium carboxymethylcellulose before use , dubbed into a suspension with a concentration of 0.1g / ml.
[0027] Experimental animals: SD male rats, weighing 200-250 g, provided by the Experimental Animal Center of Kunming Medical College, production license number SCXK (Dian) 2005-0008, use license number SYXK (Dian) 2005-0008.
[0028] Test method and results: 30 SD male rats, weighing 200-250g, were divided into 3 groups, 10 in each group. 5,6,7,4′-Tetraacetoxyflavone was infused with doses of 1g / kg, 0.5g / kg, and 0.1g / kg for 3 months, and it was detected that it had significant effects on liver and kidney function, peripheral blood, behavior, There was no obvious effect on routine urine and stool, and no abnor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com