Synthesis method of a lithium hexafluorophosphate non-aqueous solvent method
A technology of lithium hexafluorophosphate and non-aqueous solvent, applied in the field of synthesis of lithium hexafluorophosphate, can solve the problems of inability to obtain lithium hexafluorophosphate, affecting the quality of lithium hexafluorophosphate, side effects of lithium fluoride, etc., and achieve the effects of improving product quality, reducing impurities and inhibiting decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
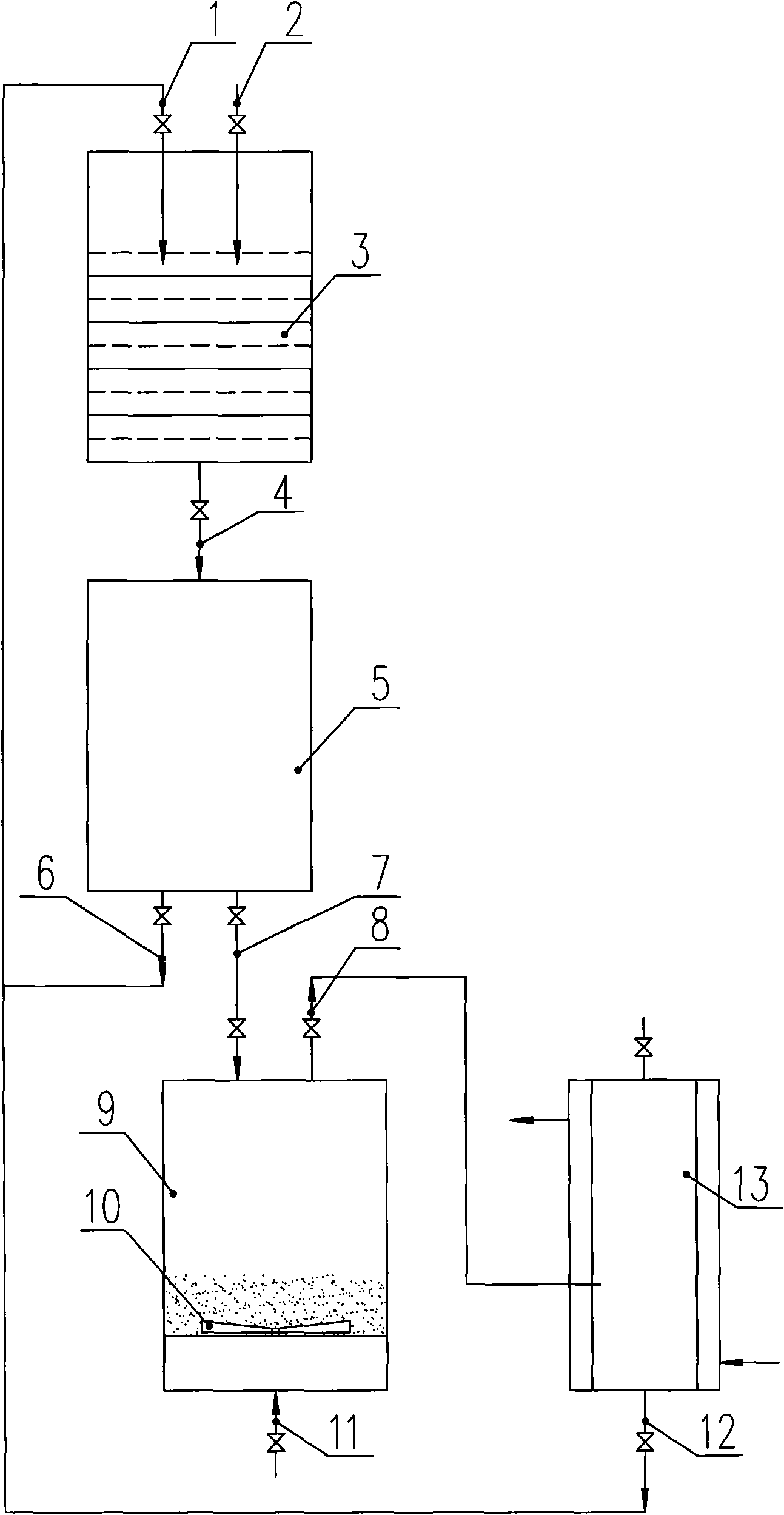
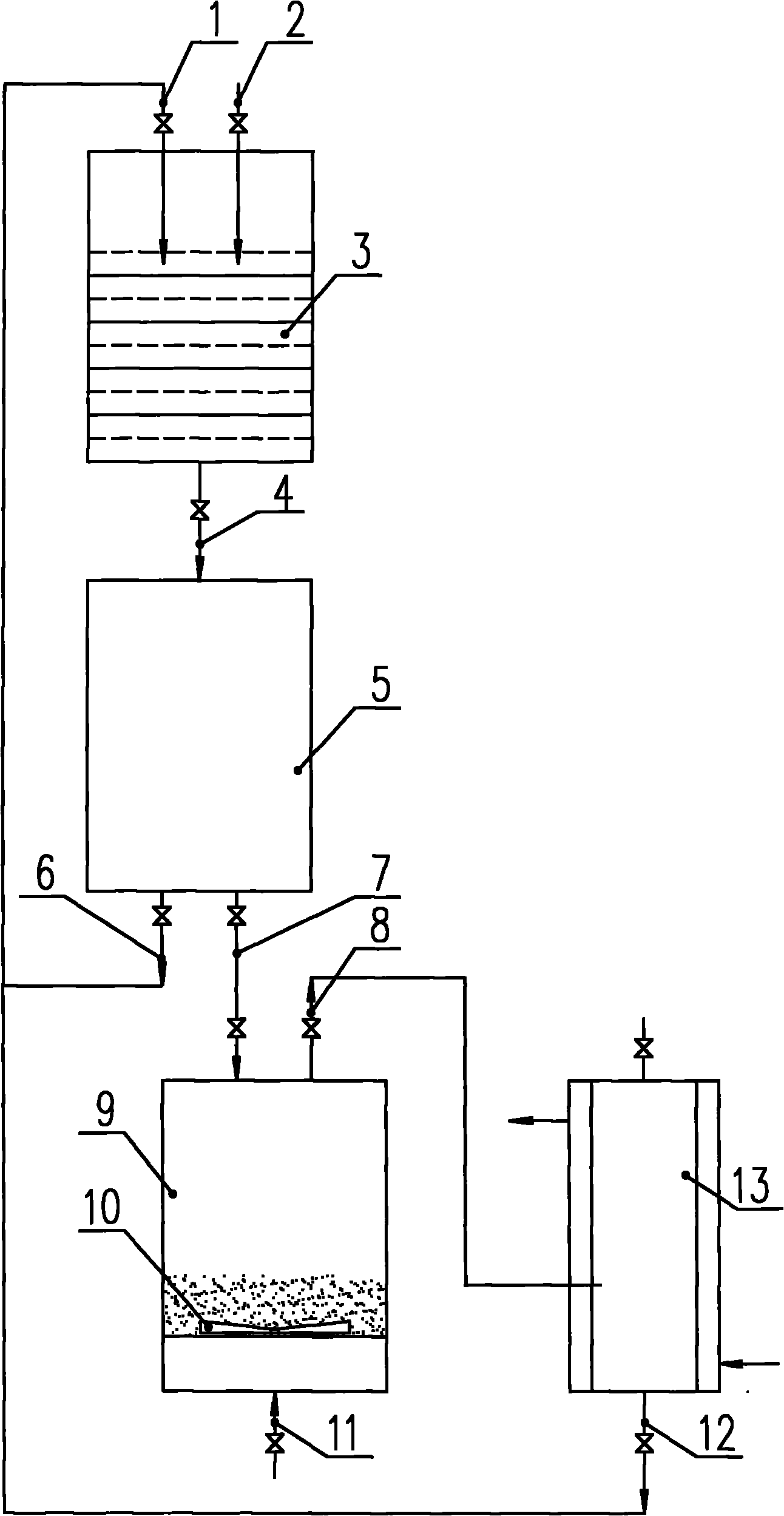
[0017] see figure 1 As shown, 50 liters of lithium fluoride anhydrous hydrogen fluoride solution with a concentration of 10% is added in advance in the reaction device 3, and gaseous phosphorus pentafluoride is passed into the reaction device 3 from the inlet 2, and the reaction temperature is controlled to be 10° C. to - 10°C, after reacting for about 240 minutes, freeze the reaction device 3 to -30°C while stirring continuously, and the reactant containing lithium hexafluorophosphate generated by the reaction is transferred from the output channel 4 to the solid product sedimentation device 5 for separation, and the separated mother liquor Through output channel 6, return to reaction device 3 by input channel 1 and continue to participate in the next batch of reactions. The solid product is transferred to drying device 9 by output channel 7. The temperature is 80 ° C and the volume ratio is nitrogen: phosphorus pentafluoride: The mixed gas of fluorine gas=96:2:2 enters the b...
Embodiment 2
[0019] see figure 1 As shown, 50 liters of lithium fluoride anhydrous hydrogen fluoride solution with a concentration of 10% is added in advance in the reaction device 3, and gaseous phosphorus pentafluoride is passed into the reaction device 3 from the inlet 2, and the reaction temperature is controlled to be 10° C. to - 10°C, after reacting for about 240 minutes, freeze the reaction device 3 to -25°C while stirring continuously, and the reactant containing lithium hexafluorophosphate generated by the reaction is transferred from the output channel 4 to the solid product sedimentation device 5 for separation, and the separated mother liquor Through the output channel 6, return to the reaction device 3 by the input channel 1 and continue to participate in the next batch of reactions. The solid product is transferred to the drying device 9 by the output channel 7. The temperature is 120 ° C, and the volume ratio is argon: phosphorus pentafluoride The mixed gas of =95:5 enters t...
Embodiment 3
[0021] see figure 1 As shown, 50 liters of lithium fluoride anhydrous hydrogen fluoride solution with a concentration of 10% is added in advance in the reaction device 3, and gaseous phosphorus pentafluoride is passed into the reaction device 3 from the inlet 2, and the reaction temperature is controlled to be 10° C. to - 10°C, after reacting for about 240 minutes, freeze the reaction device 3 to -22°C while stirring continuously, and the reactant containing lithium hexafluorophosphate generated by the reaction is transferred from the output channel 4 to the solid product sedimentation device 5 for separation, and the separated mother liquor Return to the reaction device 3 by the input channel 1 through the output channel 6 and continue to participate in the next batch of reactions. The solid product is transferred to the drying device 9 by the output channel 7. The temperature is 120 ° C and the volume ratio is argon: fluorine = 95 The mixed gas of :5 enters the bottom of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com