Method for producing chiral pure (2S,3S)-2,3-butanediol
A butanediol and chiral technology, applied in the field of production of chiral pure (2S,3S)-2,3-butanediol, can solve problems such as inability to meet the needs of industrial production, and achieve convenient product separation and simple operation process , The effect of simple culture medium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: 2S in E.cloacae ssp.dissolvens SDM, the application of 3S-BDH in producing 2S, 3S-BD
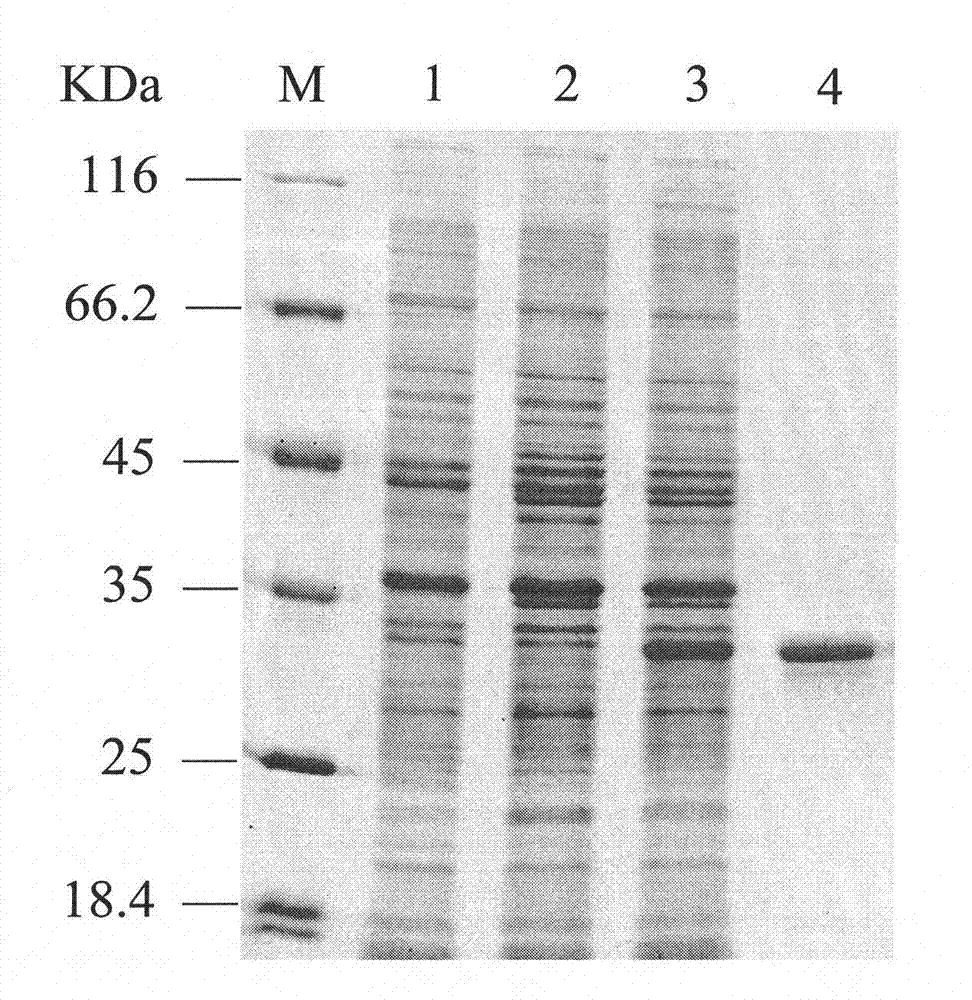
[0044] 1. Construction of recombinant genetically engineered bacteria
[0045] The genomic DNA of bacterial strain e.cloacae ssp.dissolvens SDM (CGMCC No.4230) is prepared by conventional methods, and this process can refer to the method for small-scale preparation of bacterial genomes in "Guidelines for Elaborate Molecular Biology" published by Science Press, The genomic DNA of E.cloacae ssp.dissolvens SDM was extracted; the 2S, 3S-BDH gene (budC) was amplified by PCR from the genomic DNA of E.cloacae ssp.dissolvens SDM using synthetic primers.
[0046] The source bacterium of budC gene, E.cloacae ssp.dissolvens SDM (CGMCC No.4230), is a high-yield 2,3-BD strain obtained from the preliminary screening of the applicant's laboratory, which has been preserved but not subjected to genome sequencing.
[0047] According to the sequenced Enterobacter sp.638 genome sequence, d...
Embodiment 2
[0064] Embodiment 2: 2S in K.pneumonia ATCC 49790, the application of 3S-BDH in producing 2S, 3S-BD
[0065] The method is the same as in Example 1, except that the source strain of 2S, 3S-BDH is changed to K.pneumonia ATCC 49790, and primers are designed according to the sequenced genome sequence of the bacterium as follows:
[0066] The upstream primer 5'-CCCGGATCCAATGAAAAAAGTCGCAC-3' carries a BamHI site;
[0067] The downstream primer 5'-CCCAAGCTTTTAGTTAAACACCATCCCGC-3' carries a HindIII site.
[0068] The length of the above 2S, 3S-BDH gene sequence is 771 bases, and its nucleotide sequence is shown in SEQ ID NO.2.
[0069] The whole cell of recombinant E.coli was obtained as a catalyst by the same operation as in Example 1, and the transformation reaction was carried out.
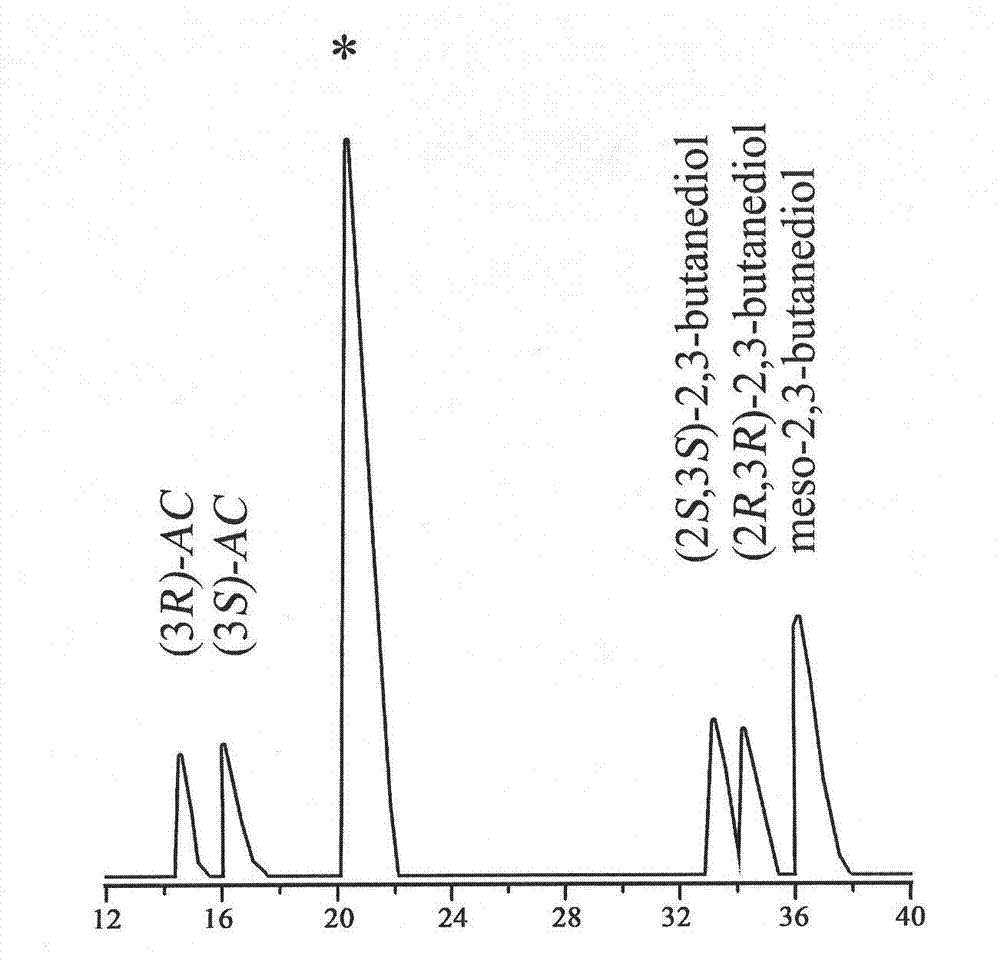
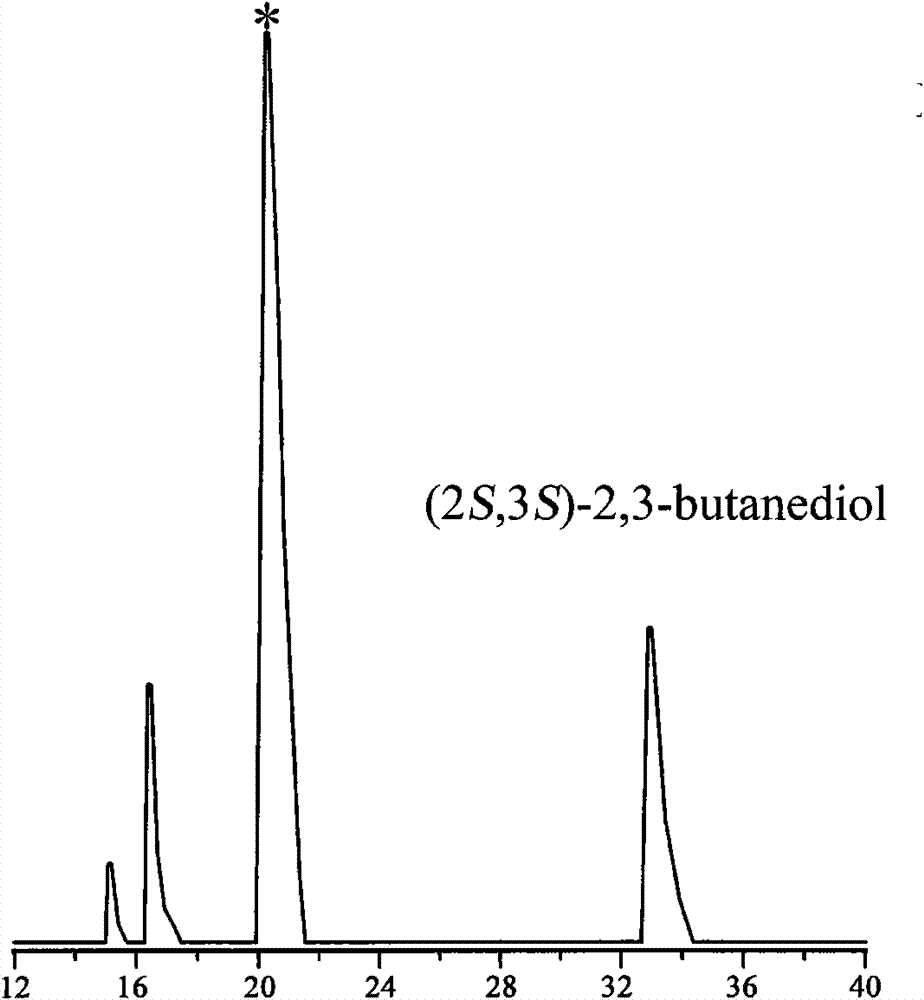
[0070] The supernatant was analyzed by gas chromatography to determine the concentration and optical purity of 2S, 3S-BD in the conversion liquid, and when the concentration of 2S, 3S-BD no longer i...
Embodiment 3
[0072] Example 3: Application of 2S in C.glutamicum ATCC 13032, 3S-BDH in the production of 2S, 3S-BD
[0073] The method is the same as in Example 1, except that the source strain of 2S, 3S-BDH is changed to C. glutamicum ATCC13032, and primers are designed according to the sequenced genome sequence of the bacterium as follows:
[0074] The upstream primer 5'-AAAGAATTCAATGAGCAAAGTTGCAATGGT-3' carries an EcoRI site;
[0075] The downstream primer 5'-TTTAAGCTTCTAGTTGTAGAGCATGCCGC-3' carries a HindIII site.
[0076] The length of the above 2S, 3S-BDH gene sequence is 777 bases, and its nucleotide sequence Genebank number is 3345609.
[0077] The whole cell of recombinant E.coli was obtained as a catalyst by the same operation as in Example 1, and the transformation reaction was carried out.
[0078] The supernatant was analyzed by gas chromatography to determine the concentration and optical purity of 2S, 3S-BD in the conversion liquid, and when the concentration of 2S, 3S-BD ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com