A spray for treating diseases caused by human papilloma viruses and a preparation method
A technology of human papillomavirus and virus infection, which is applied in the field of medicine to achieve the effect of phagocytosis activation and phagocytosis enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
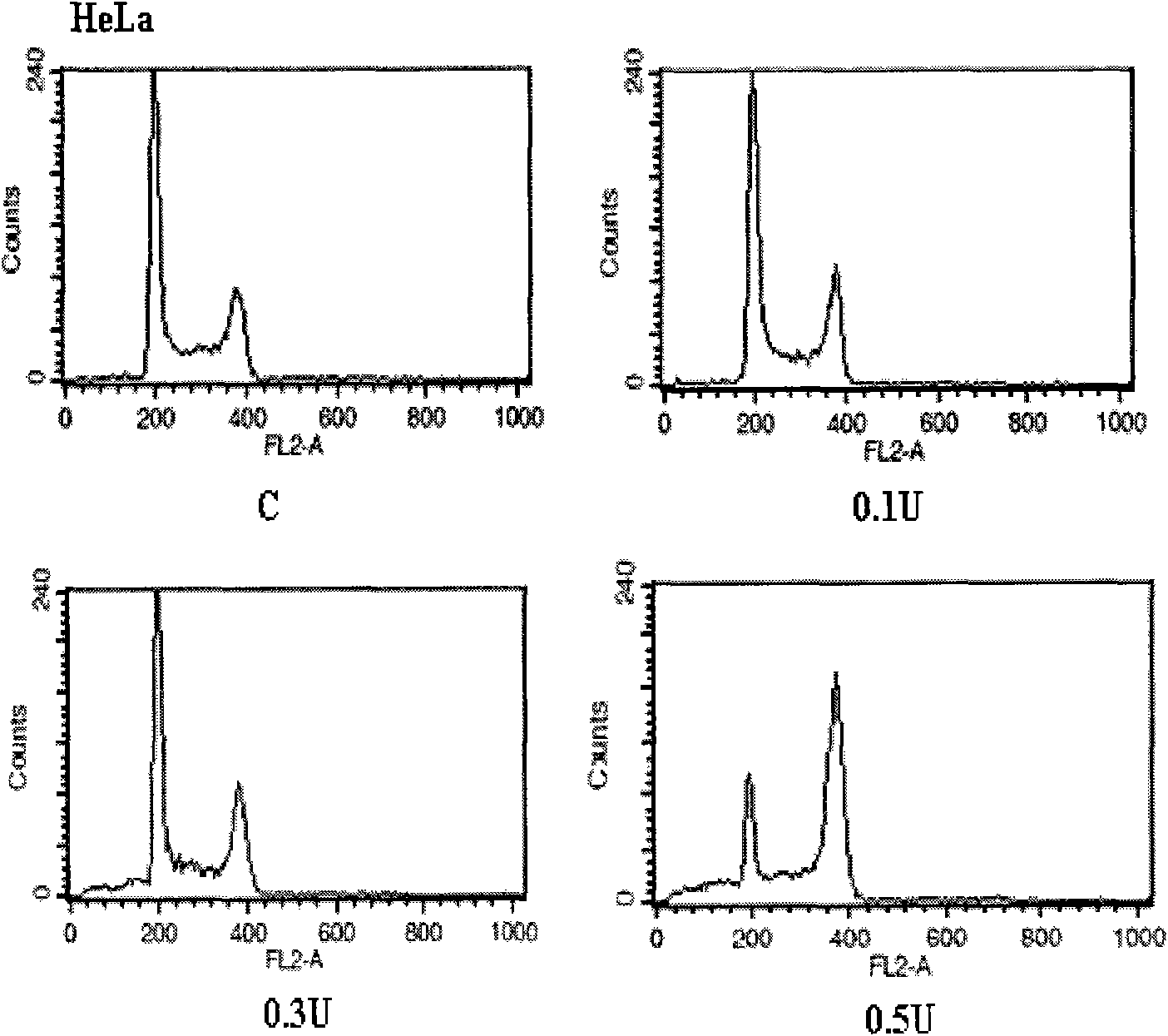
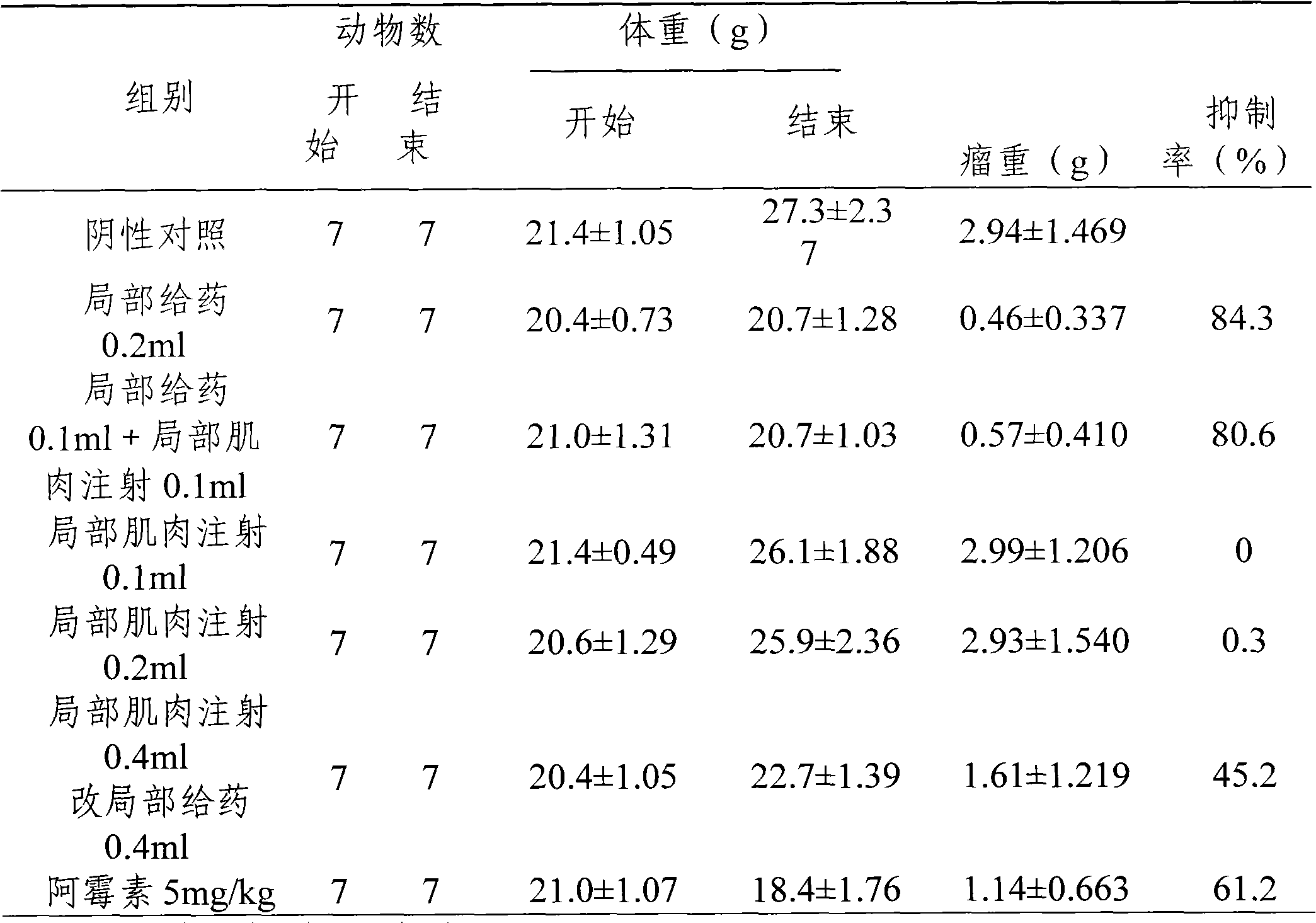
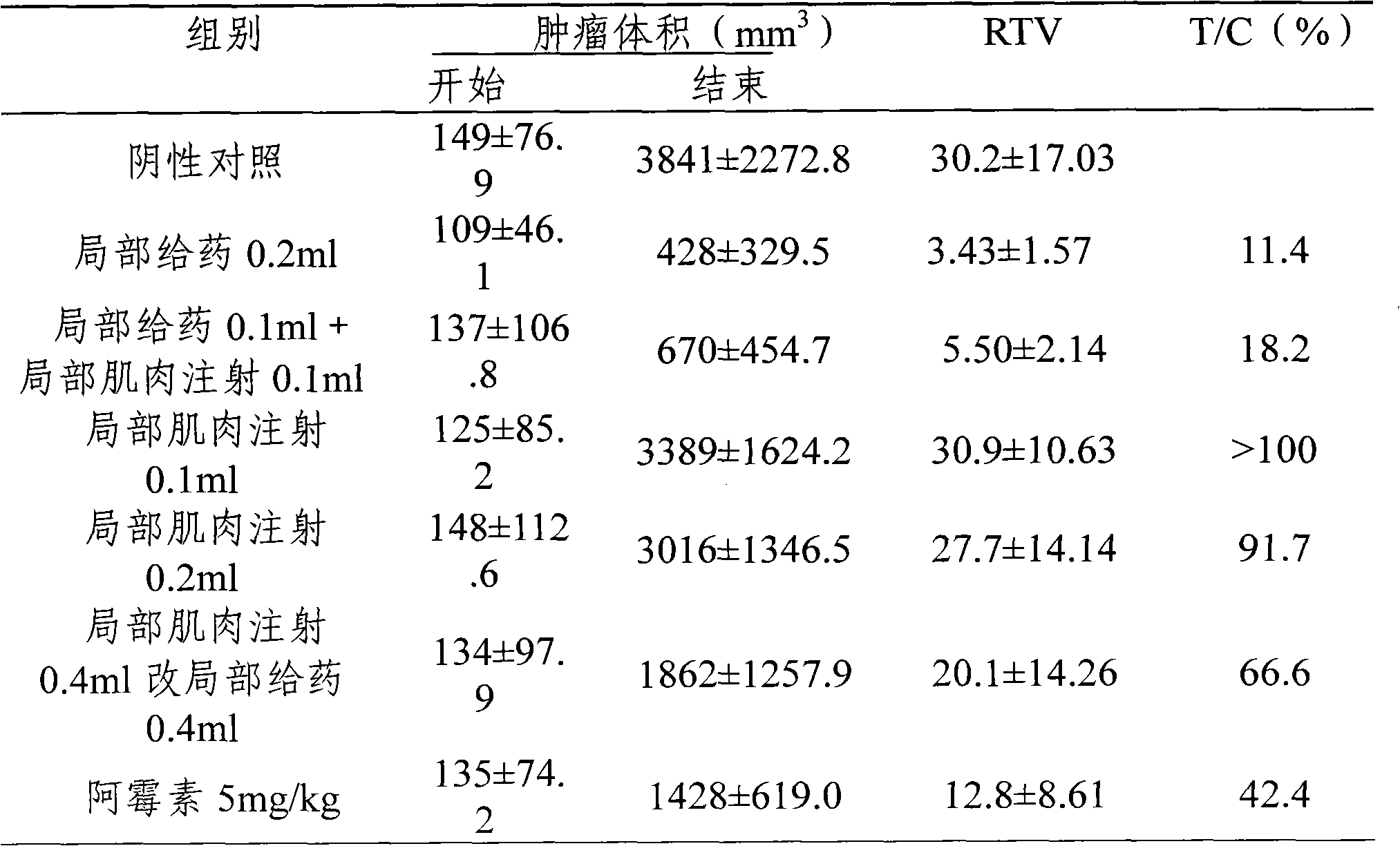
Image
Examples
Embodiment 1
[0030] Ingredients of the spray of the present invention:
[0031] According to the volume ratio, a purified human rabies vaccine with a composition of 1ml is diluted to 10ml with a mass volume concentration of 0.01g / ml chitosan-acetic acid solution.
[0032] The specific equipment method is as follows:
[0033] 1. Prepare chitosan-acetic acid solution (pH=3.2)
[0034] (1) Prepare a 0.3% acetic acid solution: add 3ml of glacial acetic acid (analytical grade) to a 1000ml volumetric flask, add pure water to the mark, where the pure water is double distilled water to obtain an acetic acid solution with a volume percentage of 0.3% ;
[0035] (2) Prepare a chitosan-acetic acid solution with a mass volume concentration of 0.01g / ml: Weigh 10g of chitosan and add it to the above acetic acid solution to completely dissolve it to 1000ml, and the solution does not allow insoluble particles or insoluble matter to form a mass Chitosan-acetic acid solution with a volume concentration of 0.01g / ml; ...
Embodiment 2
[0041] The difference from Example 1 is:
[0042] 1. Prepare chitosan-acetic acid solution (pH=4.9):
[0043] 4ml of glacial acetic acid used to prepare 0.4% acetic acid solution is (chemically pure); the pH value is adjusted to 4.9 with a sodium hydroxide solution with a molar concentration of 0.1 mol / L or an acetic acid solution with a molar concentration of 0.1 mol / L.
[0044] Prepare a chitosan-acetic acid solution with a mass volume concentration of 0.02g / ml: weigh 20g of chitosan and add it to the above acetic acid solution to completely dissolve it to 1000ml, the solution does not allow insoluble particles or insoluble matter, and the mass volume concentration is 0.02g / ml chitosan-acetic acid solution.
[0045] 2. Preparation of vaccine solution
[0046] Take 1ml of the freeze-dried purified human rabies vaccine with an activity unit of 15IU (in this example, use two, each 0.5ml, 7.5IU) and add it to a 10ml measuring flask. The preparation of the purified human rabies vaccine is...
Embodiment 3
[0048] The difference from Example 1 is:
[0049] 1. Prepare chitosan-acetic acid solution (pH=5.0):
[0050] 10ml of glacial acetic acid used to prepare 1.0% acetic acid solution is chemically pure; the pH is adjusted to 5.0 with a sodium hydroxide solution with a molar concentration of 0.1 mol / L or an acetic acid solution with a molar concentration of 0.1 mol / L.
[0051] Prepare a chitosan-acetic acid solution with a mass volume concentration of 0.03g / ml: Weigh 30g of chitosan and add it to the above acetic acid solution to completely dissolve it to 1000ml. The solution does not allow insoluble particles or insoluble matter, and the mass volume concentration is 0.03g / ml chitosan-acetic acid solution.
[0052] 2. Preparation of vaccine solution
[0053] Take 1ml of purified human rabies vaccine with an activity unit of 10IU (two in this example, 0.5ml each, 5IU) and add the solution to a 10ml volumetric flask. The purified human rabies vaccine is prepared as rabies virus CTN. Strain....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com