Human anti-CD20 monoclonal antibody and preparation method and application thereof
A fully human, antibody-based technology, applied in the biological field, can solve problems such as reduced curative effect, easy recurrence or drug resistance, and difficult to cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] Example Preparation of Antibody
[0021] (1) Cloning of human antibody light and heavy chain constant region genes
[0022] Lymphocyte separation fluid (product of Dingguo Biotechnology Development Co., Ltd.) was used to separate healthy human lymphocytes, and total RNA was extracted with Trizol reagent (product of Invitrogen Company). Research, 1982, 10: 4071-4079) respectively designed primers using RT-PCR reaction to amplify the antibody heavy chain and light chain constant region genes. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector (promega company product). After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO: 1 and SEQ ID NO: 2 show the nucleotide sequence and amino acid sequence of the heavy chain constant region (CH), respectively. SEQ ID NO: 3 and SEQ ID NO: 4 show the nucleotide sequence and amino acid sequence of the light chain constant region (CL), resp...
experiment example 1
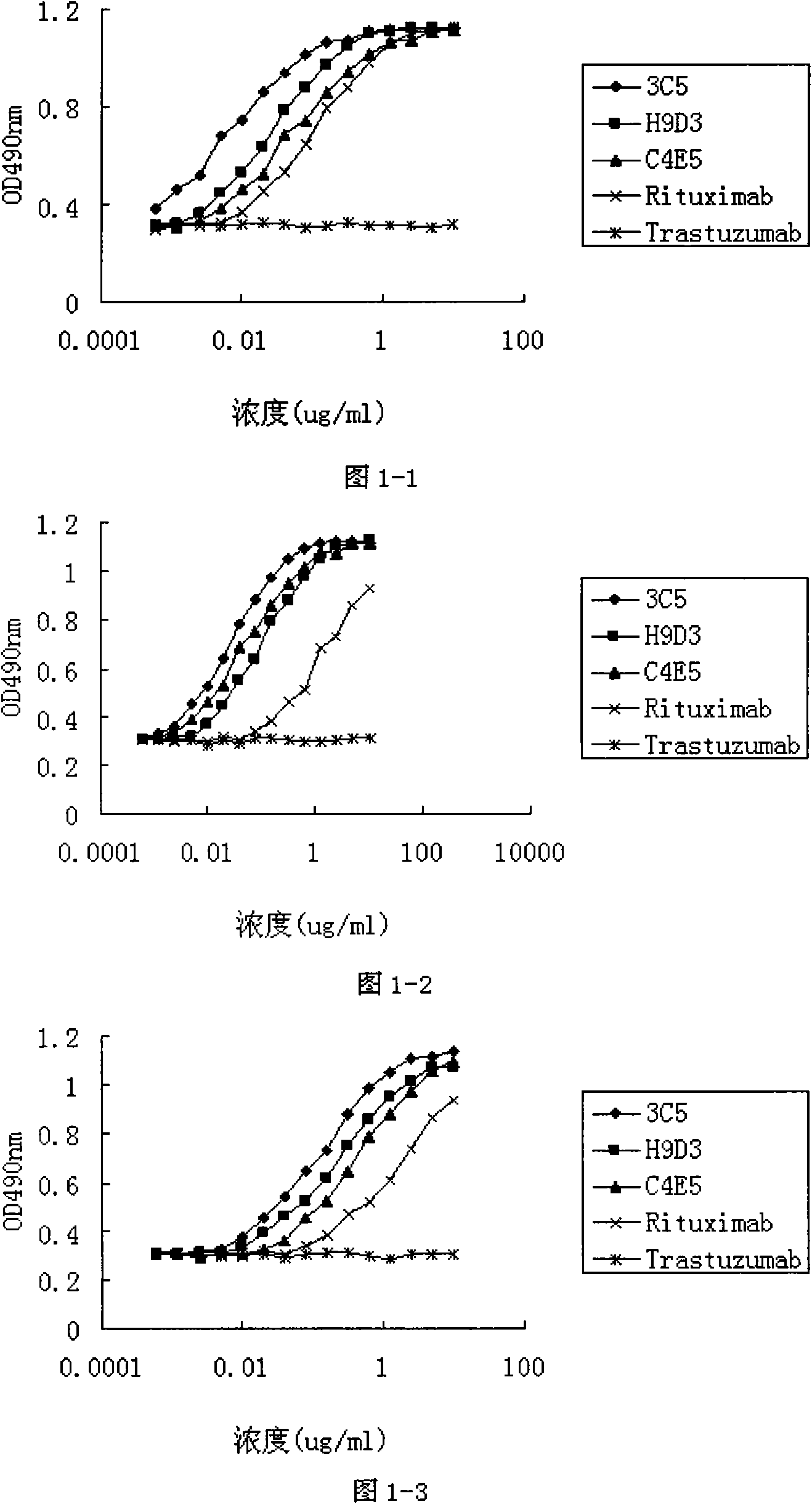
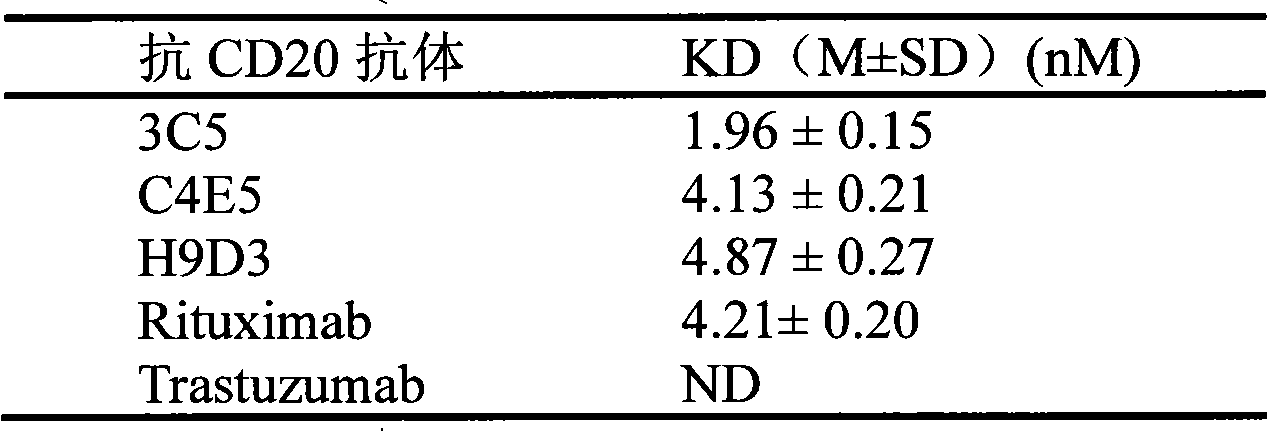
[0035] Experimental Example 1. CD20 Antibody Affinity Detection
[0036] The affinity of CD20 antibody was determined by radioimmunoassay【Cragg MS, Morgan SM, Chan HT, Morgan BP, Filatov AV, Johnson PW, French RR, Glennie MJ(2003) Complement-mediated lysis by anti-CD20mAb correlates with segregation intolipid rafts .Blood 101(3):1045-1052]. Each CD20 antibody (fully human antibody 3C5; chimeric antibody C4E5; humanized antibody H9D3; rituximab (C2B8, Roche); negative control antibody Trastuzumab Genentech) was labeled by iodobead method. The 125-iodine-labeled antibody was incubated with Daudi (ATCC CCL-213) cells for 2 hours at 37°C. Cell-bound and free iodine-labeled antibodies were separated by centrifugation, and the radioactivity of 125-iodine-labeled antibodies bound to cells was detected. The affinity constants of each CD20 antibody were obtained by Hill equation curve fitting titration binding curve (see Table 1). The KD value of the fully human antibody 3C5 was 1.9...
experiment example 2
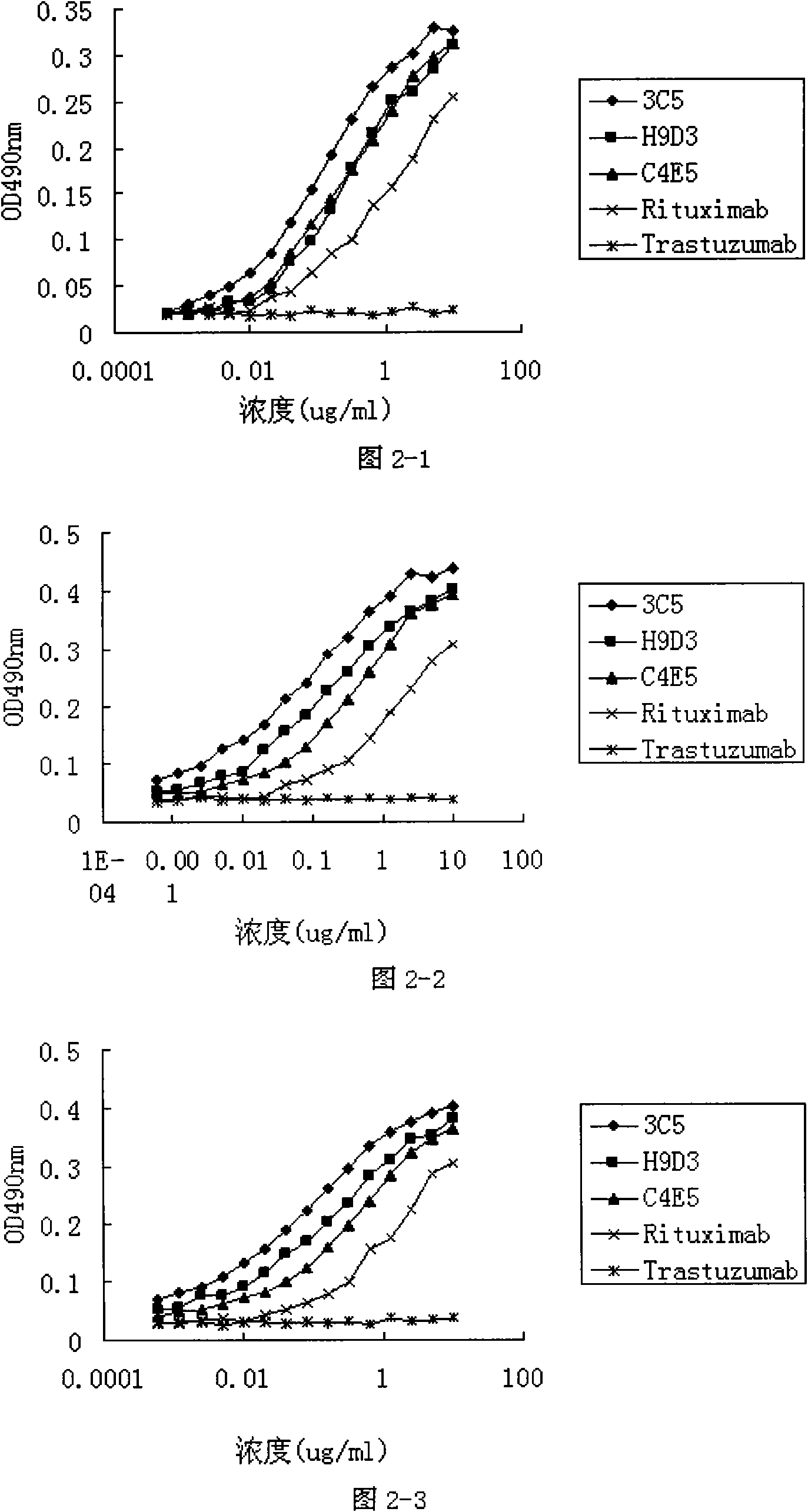
[0040] Experimental example 2. Complement-dependent killing effect (CDC) experiment of anti-CD20 antibody 3C5 on target cells
[0041] Anti-CD20 antibody can induce the killing effect of CD20 positive cells Raii, Namalwa and Daudi cells through the complement-dependent killing effect. Raji (ATCC CCL-86), Namalwa (ATCC CRL-1432) and Daudi (ATCC CCL-213) cells in the logarithmic growth phase were washed twice with PBS, and Raji, For Namalwa and Daudi cells, adjust the cell density to 2×10 5 / mL spare. Fully human anti-CD20 monoclonal antibody 3C5, humanized antibody H9D3, chimeric antibody C4E5, Rituximab (Roche) and Transtuzumab (Genentech) were diluted to a concentration of 10 μg / ml with phenol red-free RPMI-1640 culture medium, and For doubling serial dilution, the diluted samples were added to 96-well cell culture plates, 100 μl / well, and duplicate wells were set. For Raji, Namalwa and Daudi cell suspensions with adjusted densities, 5 μl of fresh human serum was added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com