Human monoclonal antibody against IgE, preparation method and purpose thereof
A monoclonal antibody, fully human technology, applied in the biological field, can solve the problems of low affinity, failure to achieve full humanization, no data on safety and effectiveness, and achieve the effect of high antibody affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Preparation of Example Antibodies
[0036] (1) Cloning of human antibody light and heavy chain constant region genes
[0037] Lymphocyte separation fluid (product of Dingguo Biotechnology Development Co., Ltd.) was used to separate healthy human lymphocytes, and total RNA was extracted with Trizol reagent (product of Invitrogen Company). Research, 1982, 10: 4071-4079) respectively designed primers using RT-PCR reaction to amplify the antibody heavy chain and light chain constant region genes. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector (promega company product). After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO: 1 and SEQ ID NO: 2 show the heavy chain constant region (C H) nucleotide sequence and amino acid sequence. SEQ ID NO: 3 and SEQ ID NO: 4 show the light chain constant region (C L ) nucleotide sequence and amino acid sequence. The correct clone in thi...
experiment example 1
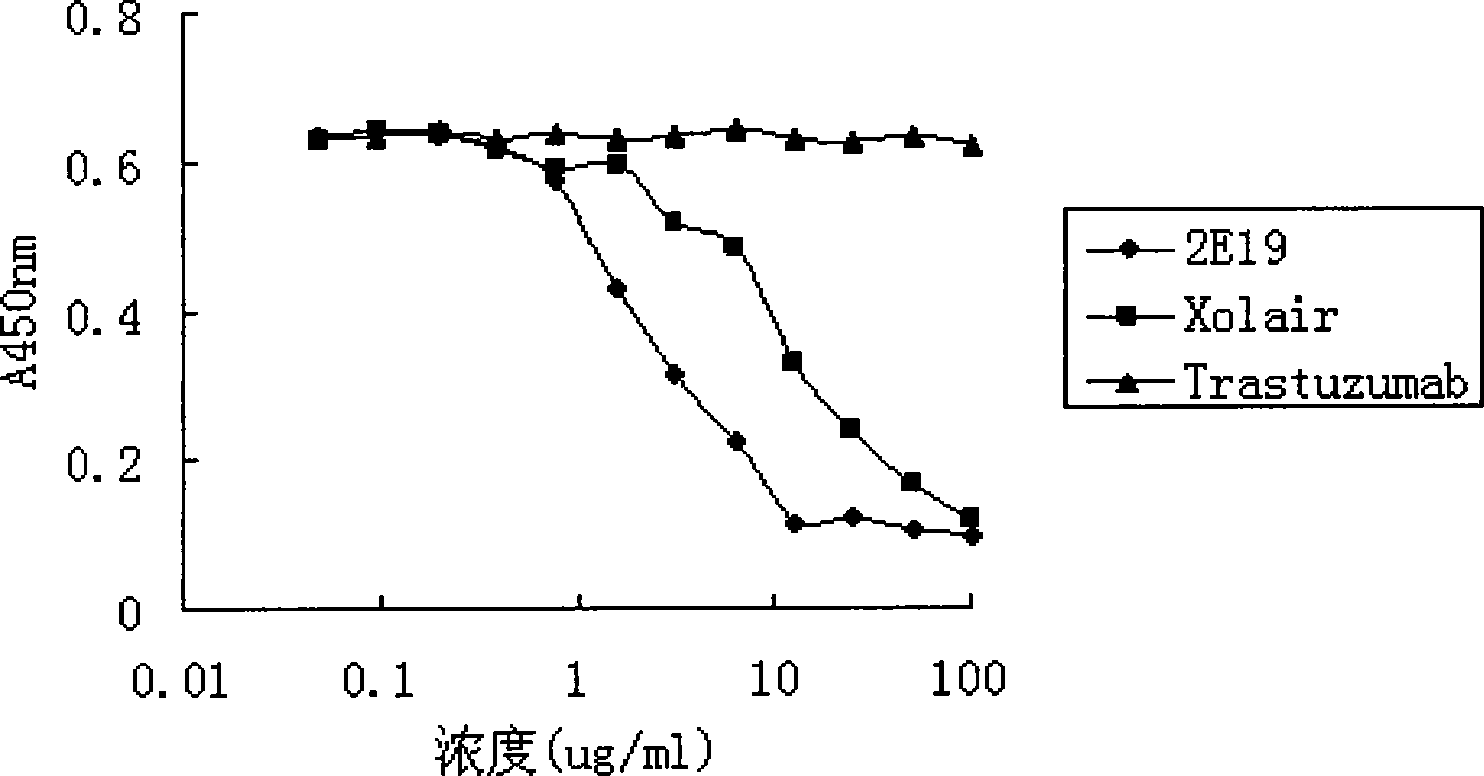
[0049] Experimental example 1. Affinity detection of anti-IgE antibody 2E19
[0050] Affinity constants of anti-IgE antibodies were detected by surface plasmon resonance (SPR) using a Biacore T100 system (Biacore AB, Uppsala, Sweden). The Fc fragment of the recombinant human IgE antibody was covalently bonded to the CM5 biosensor chip (Biacore) through the amino group, and ① fully human antibody 2E19; ② fully human antibody Xolair (Omalizumab, a commercially available product listed abroad); ③ negative control Antibody Trastuzumab (commercially available product) (in PBS / 0.05% TWEEN-20 (ICI Americas) (detergent) solution) was made into different concentrations (2-fold ratio concentration dilution), and passed through the chip at a flow rate of 50 μl / min. After each check, wash with 5 μl of 50 mM hydrochloric acid aqueous solution at a flow rate of 3 μl / min, thereby the residual antibody is eluted from the immobilized ligand. Using BIAevaluation software (T100evaluation 2.0 ver...
experiment example 2
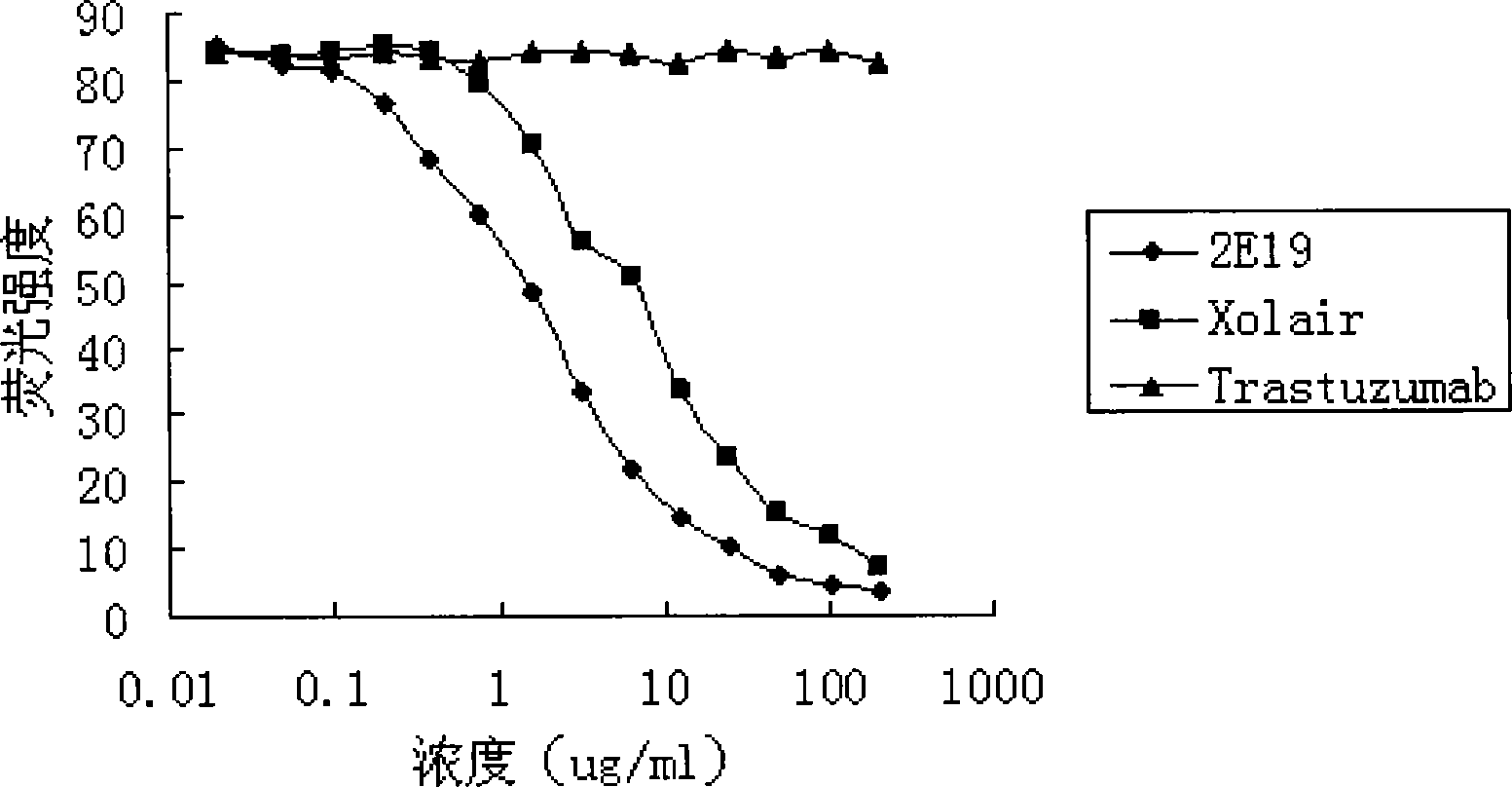
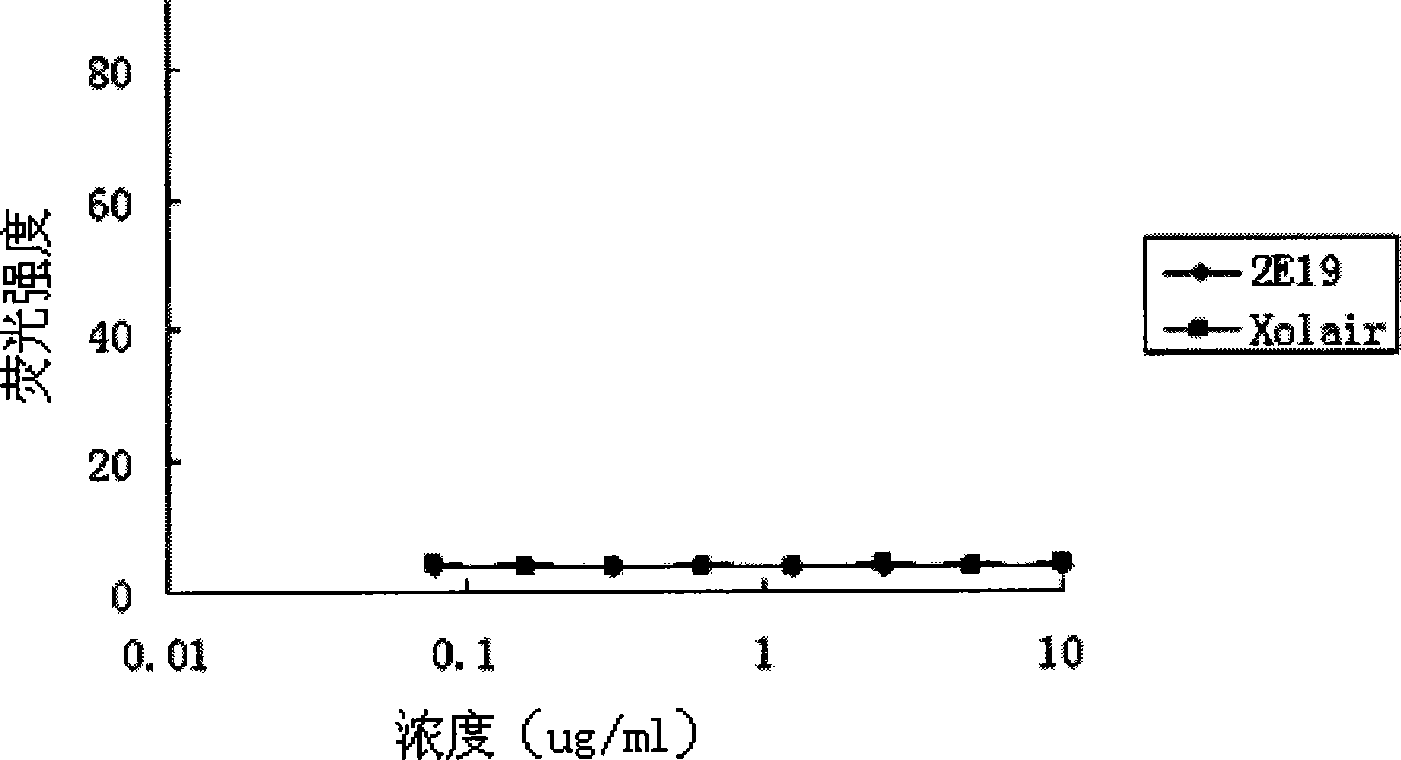
[0054] Experimental example 2. Anti-IgE antibody 2E19 inhibits the binding experiment of IgE and soluble FcεRI
[0055] Human IgE can specifically bind to its receptor FcεRI. The dose-effect curve of horseradish peroxidase (HRP)-labeled IgE binding to FcεRI should be "S"-shaped, that is, with the increase of HRP-IgE concentration, the degree of color development will gradually increase until it reaches saturation; anti-IgE antibody It can specifically bind to the receptor binding site of IgE and inhibit the binding of IgE to the corresponding receptor FcεRI. If there is anti-IgE antibody competition for HRP-IgE in the reaction system, under the condition of constant HRP-IgE concentration, with the increase of the added anti-IgE concentration, the amount of HRP-IgE bound by FcεRI will gradually decrease, so the dose-effect curve is Reverse "S" shape; IC 50 The smaller the value, the stronger the ability of the added anti-IgE antibody to compete with HRP-IgE, that is, the bett...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com