Macroporous molecular sieve, preparation method and application in pyridylmethyl amine preparation
A technology of macroporous molecular sieve and picolylamine, applied in molecular sieve compounds, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of large pollution, long reaction time, high cost, etc., and achieve high conversion rate of raw materials and short reaction time , The effect of low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
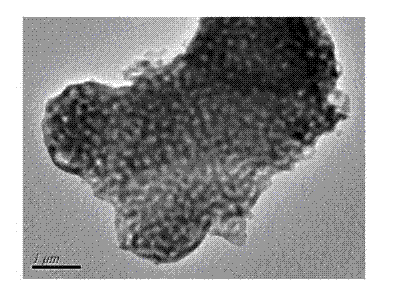
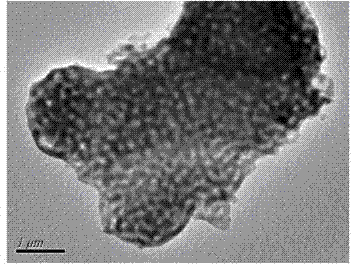
[0026] Example 1 (example of optimal reaction conditions): Dissolve 2.1840g CTAB in 50ml of water, stir for 10min to make it clear, add NaOH (0.3435g) and 10ml of water, control the pH of the solution to 9, and obtain a colorless homogeneous solution . Then slowly add 14.42gTEOS to obtain a milky white turbid liquid. After stirring and reacting at room temperature for 8h, the solution is placed in a reaction kettle and heated at 100 0 C reacted for 3 days. Suction filtration, washed twice with ethanol and water respectively to obtain a white solid, the white solid 550 0 C, burn in the muffle furnace for 6h, 750 0 Calcined in C for 11 hours to obtain macroporous molecular sieve (MMCM), 11.6 g, yield 78%. Infrared spectral data (IR, cm -1 ): 1089, 800, 432. BET: 21.54m 2 g ?1 ; pore size is 100 nm (see attached figure 1 )
Embodiment 2
[0027] Example 2: Dissolve 2.1840g of CTAB in 50ml of water, stir for 10min to make it clear, and then add NaOH (0.3435g).
[0028] and 10ml of water to control the pH of the solution to 8 to obtain a colorless homogeneous solution. Then slowly add 14.42gTEOS to obtain a milky white turbid liquid. After stirring and reacting at room temperature for 8h, the solution is placed in a reaction kettle and heated at 100 0 C reacted for 3 days. Suction filtration, washed twice with ethanol and water to obtain a white solid, the white solid 650 0 C, burn in the muffle furnace for 6h, 900 0 Calcined in C for 11 hours to obtain macroporous molecular sieve (MMCM), 8.6 g, yield 58%. Infrared spectral data (IR, cm -1 ): 1089, 800, 432.
Embodiment 3
[0029]Example 3: Dissolve 2.1840g of CTAB in 50ml of water, stir for 10min to make it clear, add NaOH (0.3435g) and 10ml of water, control the pH of the solution to 10, and obtain a colorless homogeneous solution. Then slowly add 14.42gTEOS to obtain a milky white turbid liquid. After stirring and reacting at room temperature for 8h, the solution is placed in a reaction kettle and heated at 100 0 C reacted for 3 days. Suction filtration, washed twice with ethanol and water respectively to obtain a white solid, the white solid 550 0 C, burn in the muffle furnace for 6h, 800 0 C burned for 11 hours to obtain macroporous molecular sieve (MMCM), 7.8 grams, yield 53%. Infrared spectral data (IR, cm -1 ): 1089, 800, 432.
[0030] Preparation of 2-picolylamine
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com