Sustained-release microspheres and preparation method thereof
A slow-release microsphere and slow-release technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of incomplete release and inability to overcome the encapsulation rate, and achieve Good uniformity, regular particles without adhesion, and good redispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
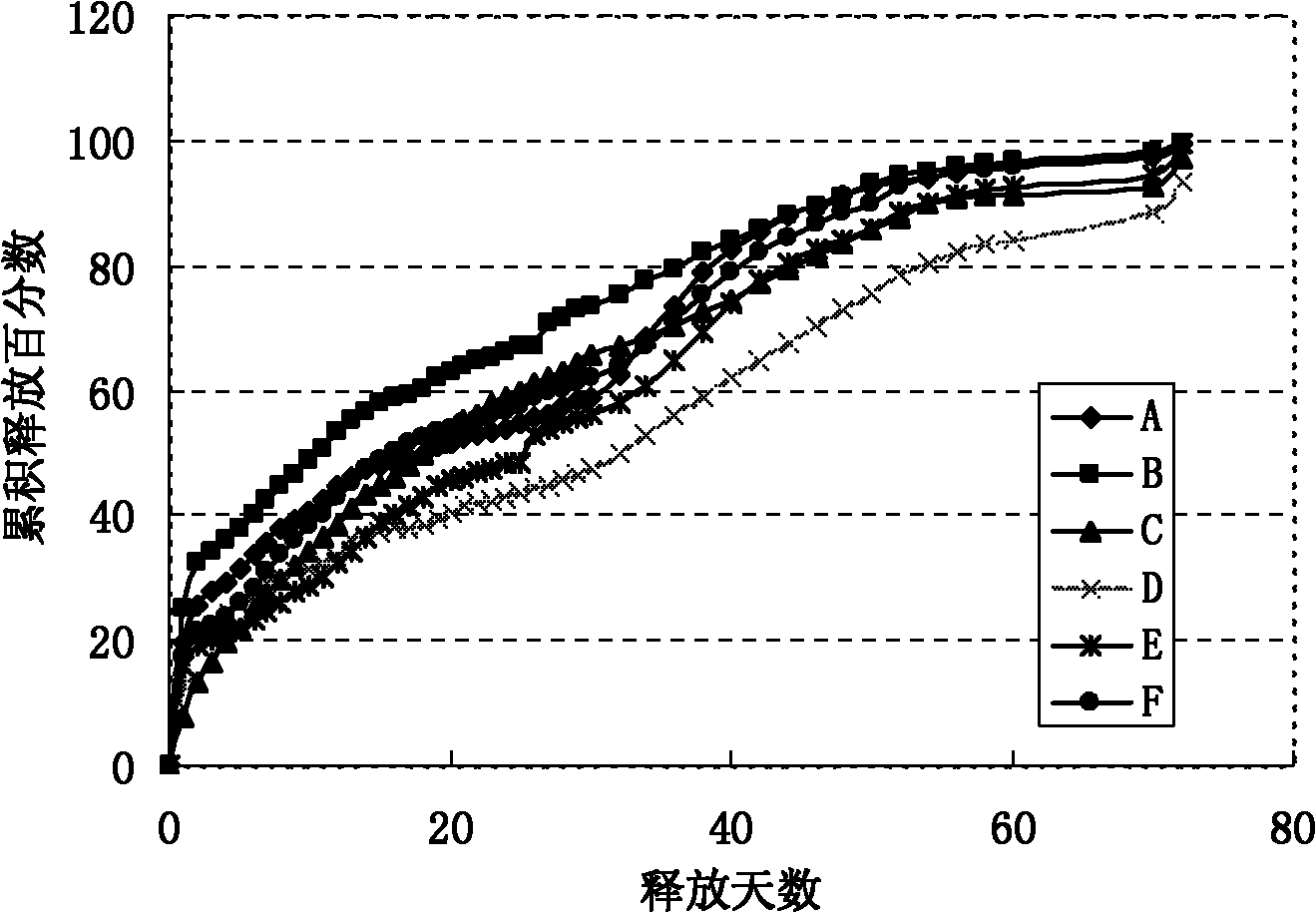
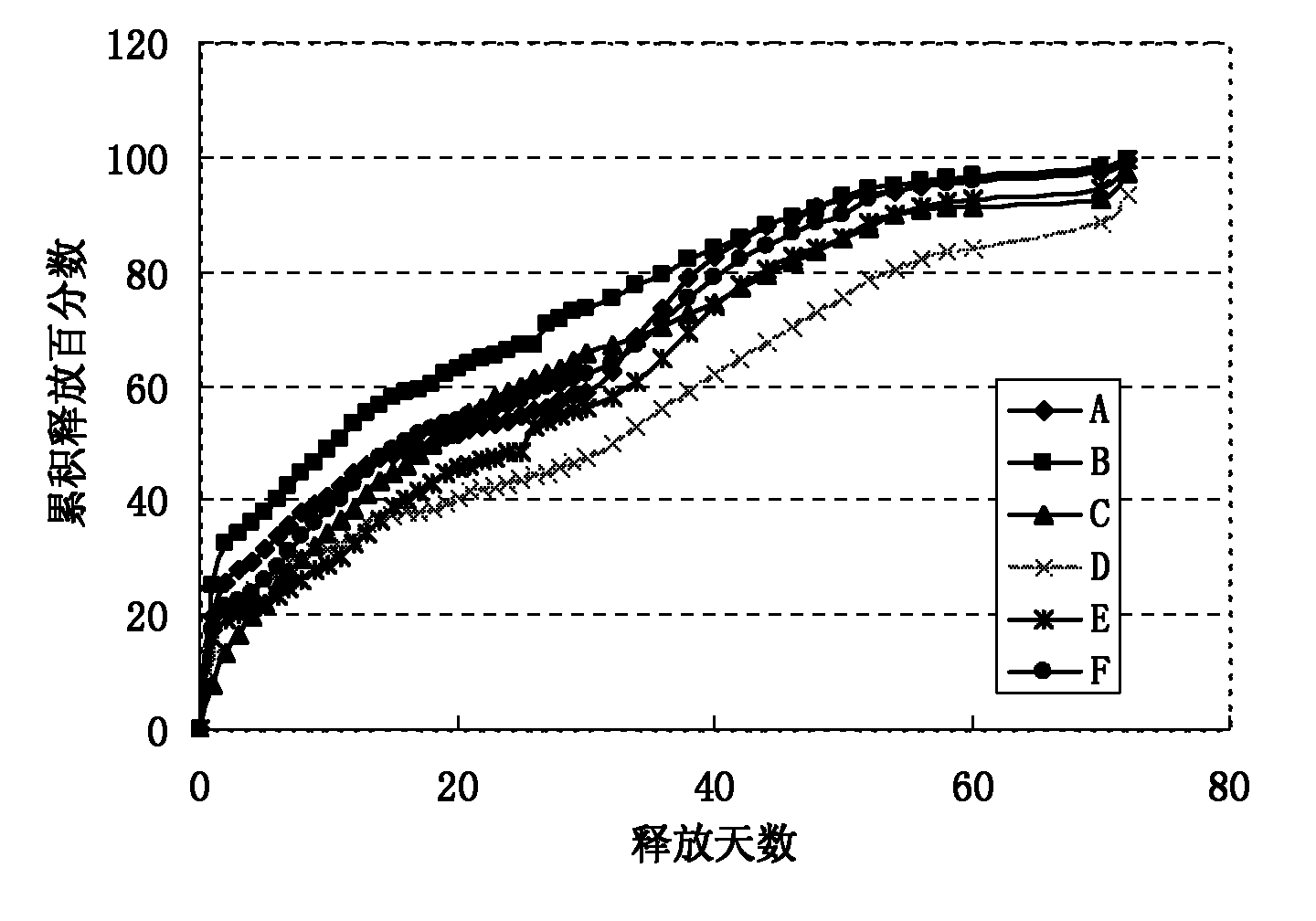
[0025] The present invention addresses the problems of low encapsulation rate, incomplete release, severe burst release, and easy environmental pollution in the current preparation method of sustained-release microspheres, and provides a method for preparing sustained-release microspheres. A new preparation process is adopted: the preparation technology of hydrophilic oil-in-water-hydrophilic oil-in-oil-solid-in-oil (S / O / hO / W), which specifically includes the following steps:
[0026] A. Add the embedded solid components to the organic solution of the slow-release or controlled-release material, that is, the oil phase, and mix to form a suspension;
[0027] B. Add the suspension of step A to the hydrophilic organic solution that does not dissolve the sustained or controlled release material of step A, that is, the hydrophilic oil phase, and stir to form microspheres;
[0028] C. Disperse the microspheres formed in step B into the water phase and solidify to obtain the sustained-relea...
Embodiment 1
[0039] Preparation of blank microspheres:
[0040] (1) Preparation of oil phase (O) and hydrophilic oil phase (hO)
[0041] Oil phase (O): The controlled-release or sustained-release material is dissolved in an organic solvent to prepare a concentration of 1-50% by weight to form the oil phase (O).
[0042] Hydrophilic oil phase (hO): Formulation 1: Use surfactants and sodium chloride and other salts to mix the aqueous solution, their weight percentage concentrations are respectively 1-10% and 0-10%, and the weight percentage of the hydrophilic oil phase is 0 -40%; ethanol, ethylene glycol, propylene glycol or normal temperature liquid polyethylene glycol is 60-100%; or formula 2: use surfactant and sodium chloride and other salts to mix the aqueous solution, and their weight percentage concentration is 1- 10% and 0-10%, the weight percentage of the hydrophilic oil phase is 0-40%; the weight percentage is 0-50% glycerol and 50-100% ethanol, ethylene glycol, propylene glycol or norma...
Embodiment 2
[0048] Preparation of polylactic acid-polyglycolic acid (PLGA) microspheres containing erythropoietin dextran particles:
[0049] (1) Mix 10 mg of erythropoietin dextran particles (EPO) (with a particle size of 1-5 μm) and 100 mg of a dichloromethane solution of PLGA with a concentration of 20% by weight, stir, vortex or ultrasonic 0.5- 5 minutes to form a uniform suspension, that is, a solid-in-oil (S / O) emulsion;
[0050] (2) Add the emulsion obtained in step (1) dropwise to the hydrophilic oil phase (hO): [Preparation of the hydrophilic oil phase (hO): mix the aqueous solution with polyvinyl alcohol (PVA) surfactant and sodium chloride salt Account for 10% (5% by weight of polyvinyl alcohol, 5% by weight of sodium chloride salt); 90% by weight of ethylene glycol] and stir, vortex or ultrasonic for 0.5-5 minutes to form double emulsion;
[0051] (3) Add the double emulsion of step (2) dropwise to the water phase (W) [preparation of the water phase (W): 5% sodium chloride aqueous s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com