Binary amorphous alloy catalyst and preparation method and use thereof
A technology of amorphous alloys and catalysts, applied in the field of binary amorphous alloy catalysts, preparation of catalysts, and amorphous alloy catalysts, can solve problems that have not been reported before, and achieve high conversion rate and selectivity, The effect of excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) 0.66g InCl 3 4H 2 O was dissolved in 5.0 mL deionized water, and stirred thoroughly to obtain a 0.45 mol / L metal salt solution.
[0023] 2) Add 1.1g KBH at 10°C 4 Dissolve in 10mL deionized water, add dropwise to the above metal salt solution and stir rapidly (800rpm), black particles are gradually formed in the solution, and gas comes out.
[0024] 3) The black particles formed above were washed successively with water and absolute ethanol for 3 to 5 times respectively to obtain an In-B amorphous alloy sample.
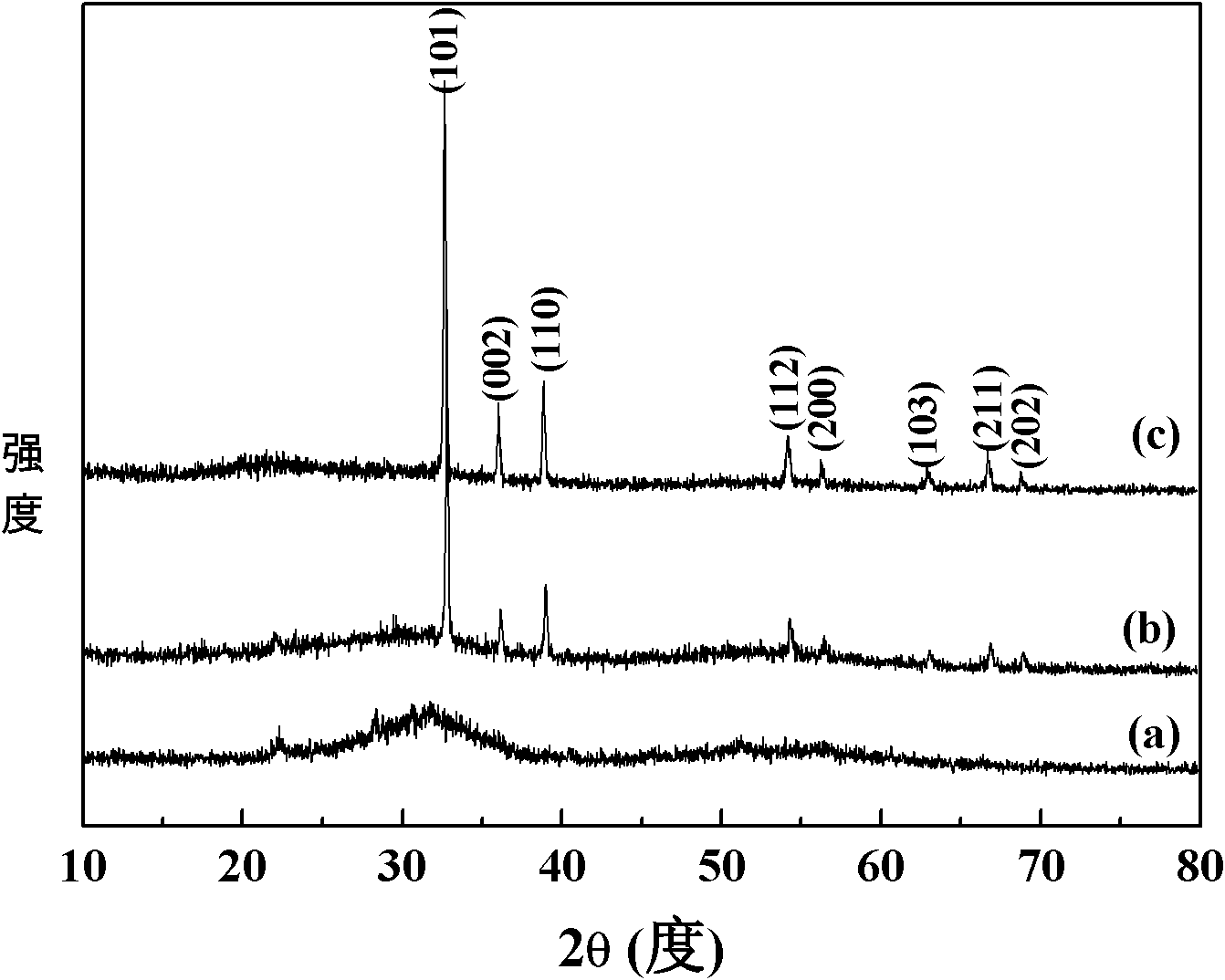
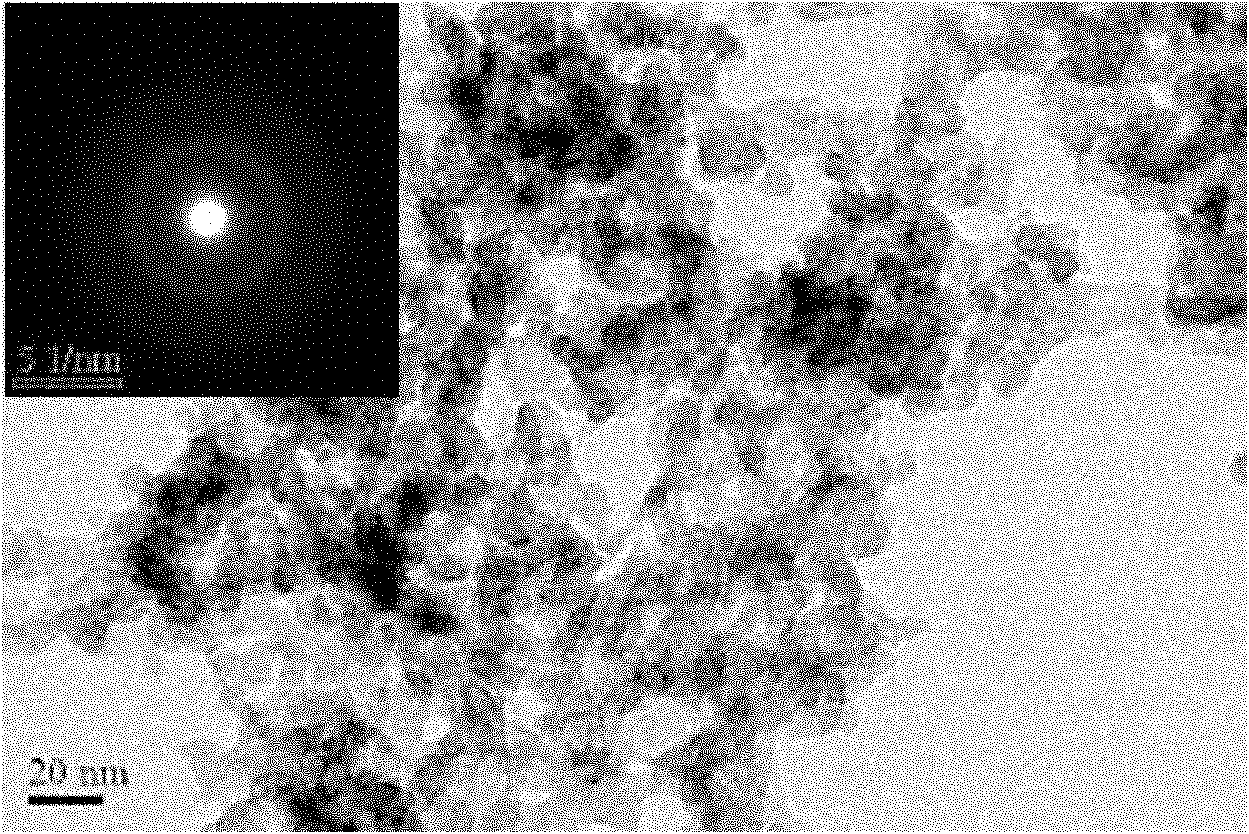
[0025] figure 1 For the XRD spectrum of the sample prepared in this example, from the comparison of a and c, it is found that the prepared sample has no crystalline characteristic peaks, and after crystallization at 200 ° C, the characteristic diffraction peaks of metal In appear, slowly stir (500rpm) Some crystallization peaks appeared in the samples prepared under the above conditions; figure 2 For the TEM photo of the sample prepared in this embodim...
Embodiment 2
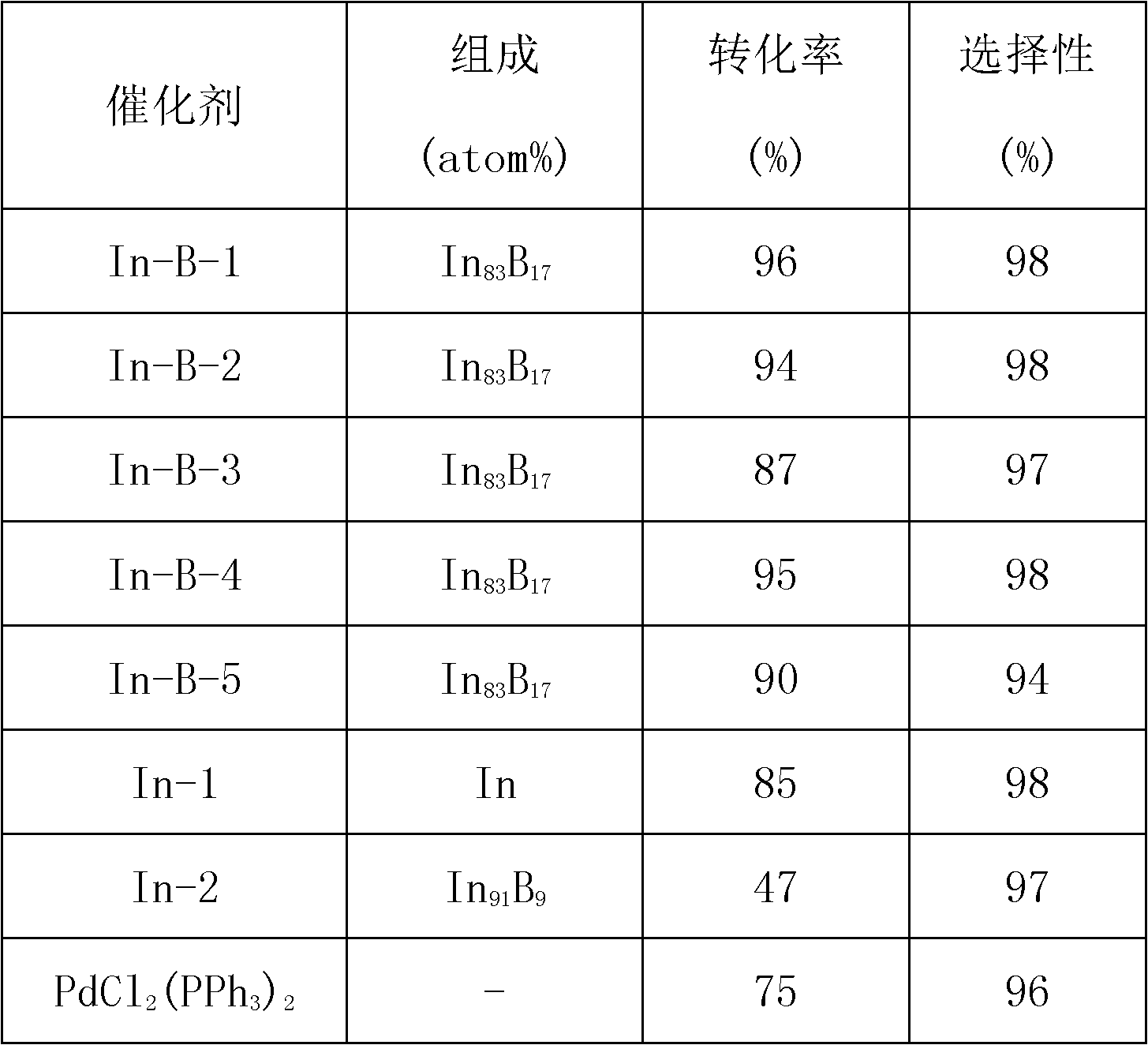
[0028] Catalyst (represented by In-B-2) prepared in Example 1 is used for the preparation of allyl of p-methyl 1-phenyl-3-butenyl-1-alcohol with respect to methyl benzaldehyde and allyl bromide Alkylation reaction, that is: 1.1mmol In-B catalyst, 0.54g aluminum powder, 2.3mmol p-tolualdehyde, 4.5mmol allyl bromide and 10mL deionized water were added in sequence in a 50mL flask, sealed and heated at a constant temperature. The required reaction temperature is 50° C.; the reaction time is 2.0 h. The separation and analysis of the reaction product are the same as in Example 1. The catalytic performance is shown in Table 1.
Embodiment 3
[0030] The catalyst (represented by In-B-3) prepared in Example 1 is used for the allylation of p-chlorobenzaldehyde and allyl bromide to prepare p-chloro 1-phenyl-3-butenyl-1-alcohol Reaction, that is: add 1.1mmol In-B catalyst, 0.54g aluminum powder, 2.3mmol p-chlorobenzaldehyde, 4.5mmol allyl bromide and 10mL deionized water in a 50mL flask in sequence, heat and keep the temperature at the required reaction The temperature was 50° C.; the reaction time was 2.0 h. The separation and analysis of the reaction products were the same as in Example 1. The catalytic properties are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com