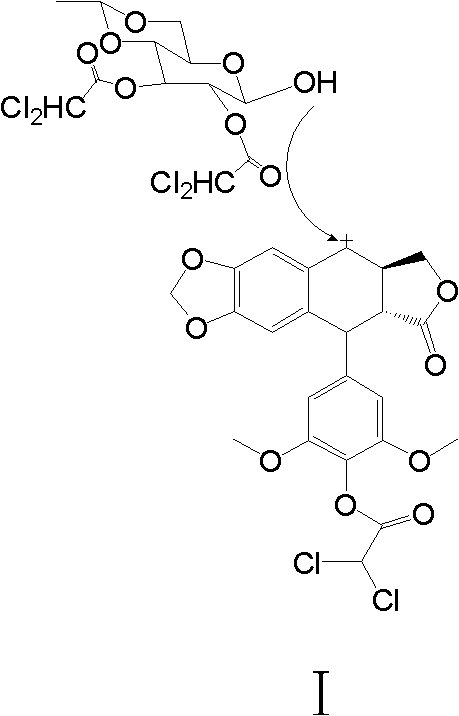
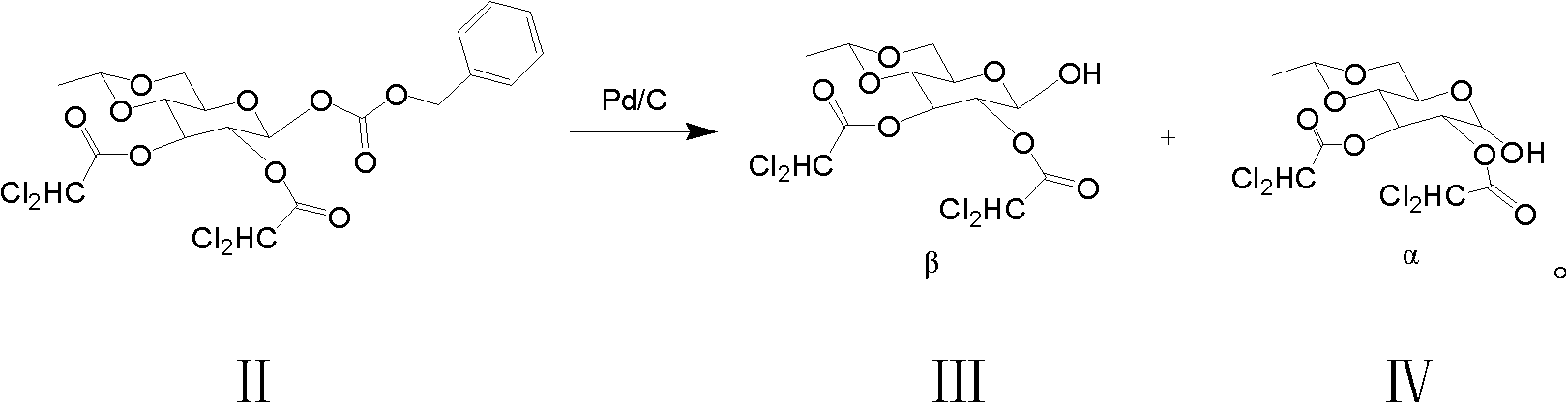
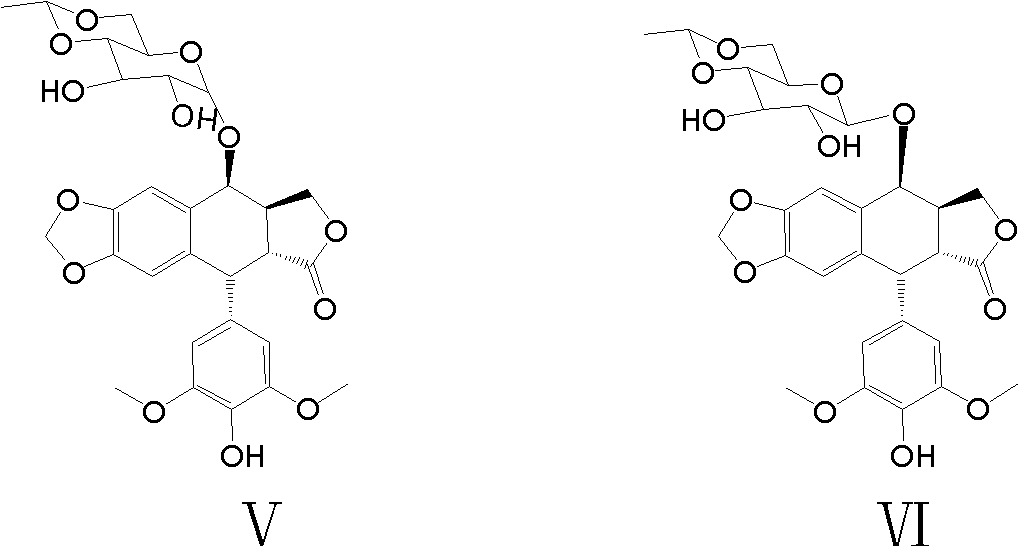
Preparation method of etoposide
A technology of etoposide and structural formula, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of increasing difficulty in purification of etoposide, increasing production costs, and many impurities, and achieving less The difficulty of purification, the effect of improving yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Structural formula is the preparation of β-glucoside of (2):
[0030] Take the compound (250g, 545mmol) of structural formula (1) and add it to a 5L four-necked reaction flask, then add 3.0L ethyl acetate and Pd / C (30g), install a hydrogen balloon, replace the hydrogen gas 3 times, and cool the reaction solution When the temperature is lower than -10°C, TLC detects that the reaction raw materials are completely consumed, filter off Pd / C, concentrate ethyl acetate under reduced pressure to about 500mL, take 2.0L of petroleum ether and add it to a 5L beaker, stir vigorously, and pour the concentrate into In petroleum ether, a large amount of white powdery solids were precipitated, filtered after stirring for 20 minutes, and dried at 45° C. to obtain 168 g (518 mmol) of β-glucoside with structural formula (2), with a yield of 95.1%. The purity of β-glucoside detected by HPLC was 95%. %, α-glucoside is 0.8%.
[0031] 2. Coupling reaction
[0032] Take 1,2-dichloroethan...
Embodiment 2
[0036] 1. Structural formula is the preparation of β-glucoside of (2):
[0037] Take the compound (500g, 1.09mol) with structural formula (1) and add it to a 10L four-necked reaction flask, then add 6.0L ethyl acetate and Pd / C (60g), install a hydrogen balloon, and replace the reaction solution with hydrogen for 3 times. Cool to below -10°C, TLC detects that the reaction raw materials are completely consumed, filter off Pd / C, concentrate ethyl acetate under reduced pressure to about 1L, take 4.0L of petroleum ether and add it to a 10L beaker, stir vigorously, pour the concentrate Put into petroleum ether, a large amount of white powdery solids precipitated, stirred for 20 minutes, filtered, and dried at 45°C to obtain 331g (1.02mol) of β-glucoside with structural formula (2), the yield was 93.8%, and the purity of β-glucoside was detected by HPLC 94.5% and 0.7% for α-glucoside.
[0038] 2. Coupling reaction
[0039] Take 1,2-dichloroethane (6.0L) and pour it into a 10L four-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com