Condensation derivative of chitosan and substituted phenylthiosemicarbazide and preparation method thereof
A technology of thioanisidine and chitosan, which is applied in the field of marine chemical engineering, can solve problems such as the gap in antibacterial activity, and achieve the effects of improving biological activity, being easy to absorb, and overcoming poor solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Derivative 1
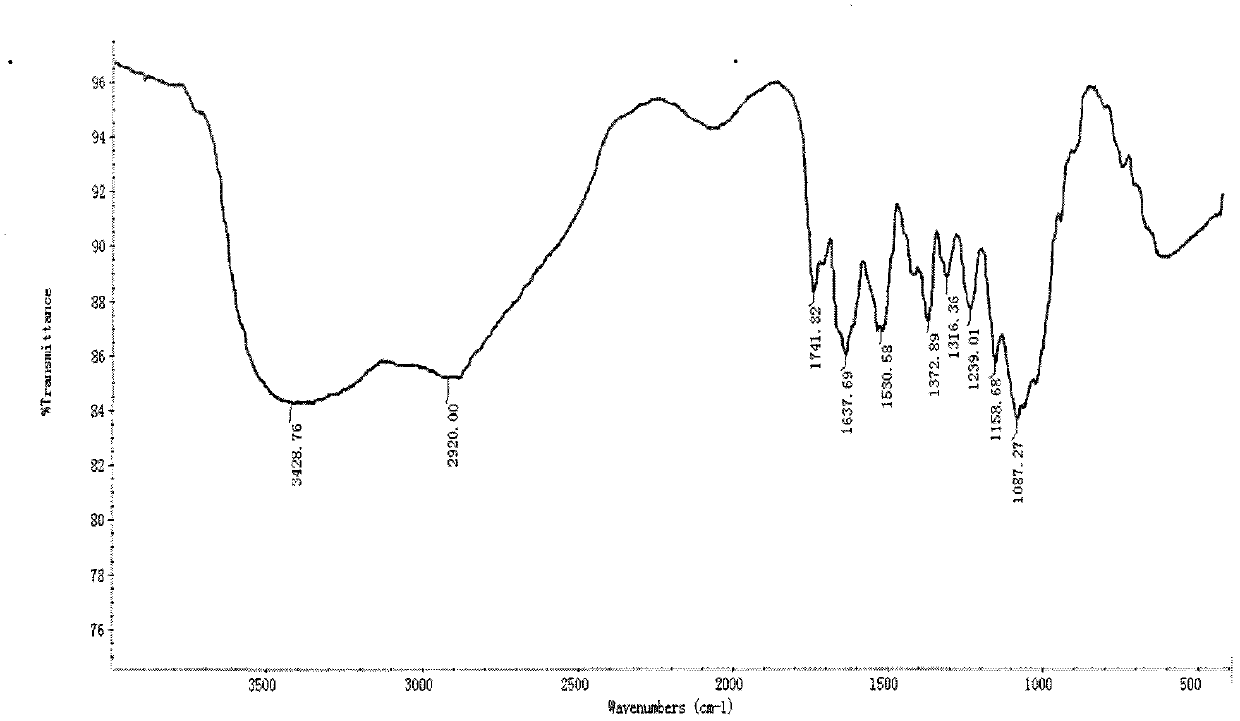
[0039] 9.99 grams of acetylated chitosan with a molecular weight of 230,000 and 23.80 grams of o-toluidine thiourea were added to 300 mL of absolute ethanol, 2.5 mL of acetic acid was added dropwise to it under stirring, and the reaction was refluxed for 8 hours, cooled to room temperature, The reactant was suction-filtered, washed with absolute ethanol, and dried at 60°C to obtain an off-white powder, namely acetylated chitosan-o-toluenethiosemicarbazide, ie derivative 1, see Table 1 for the structural formula.
[0040] The preparation of described acetylated chitosan refers to the following documents:
[0041] 1. Fujii S, Kumagai H, Noda M. Preparation of poly(acy1) chitosans [J]. Carbohydr Res, 1980, 83(2): 389-393.
[0042] 2. Huang Liyao, Liu Chao. Preparation of hydrophobized long fatty chain acylated chitosan [J]. Journal of Huaqiao University (Natural Science Edition), 2005, 26(4): 439-441.
[0043] Infrared spectrum sho...
Embodiment 2
[0044] Example 2 Preparation of Derivative 2
[0045] 2.0 grams of acetylated chitosan with a molecular weight of 230,000 and 3.87 grams of o-chloroanilinothiourea were added to 100 mL of absolute ethanol, and 0.5 mL of acetic acid was added dropwise to it under stirring, and refluxed for 8 hours, cooled to room temperature, and reacted The product was suction filtered, washed with absolute ethanol, and dried at 60°C to obtain a yellow powder, which was acetylated chitosan condensed o-chlorophenylthiosemicarbazide, namely derivative 2. See Table 1 for the structural formula.
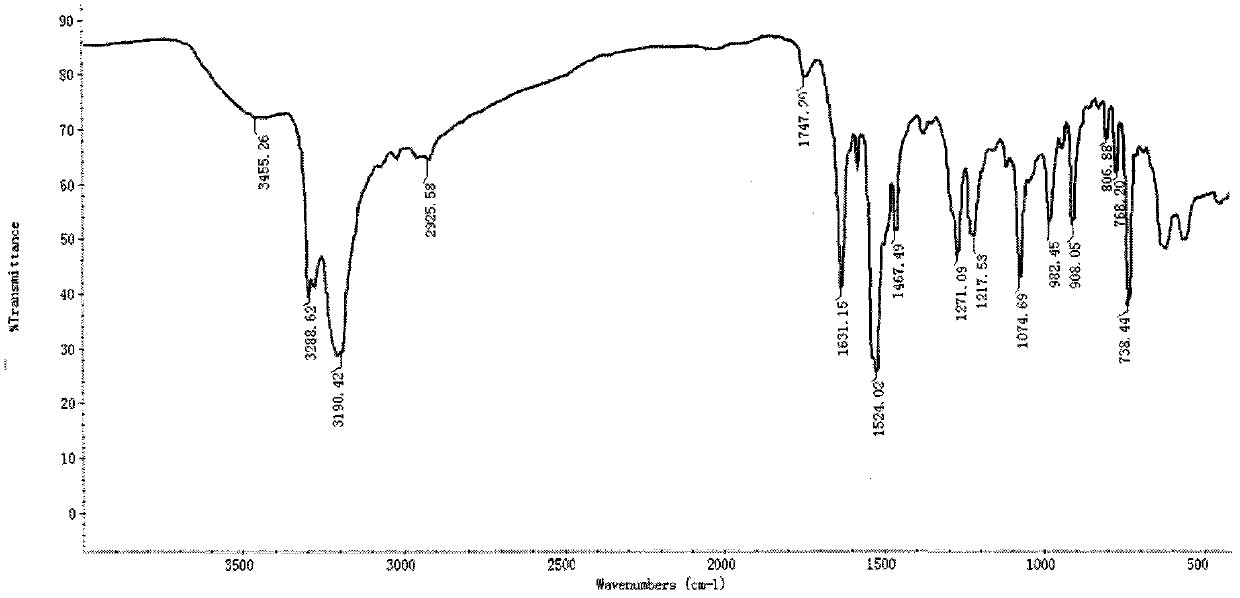
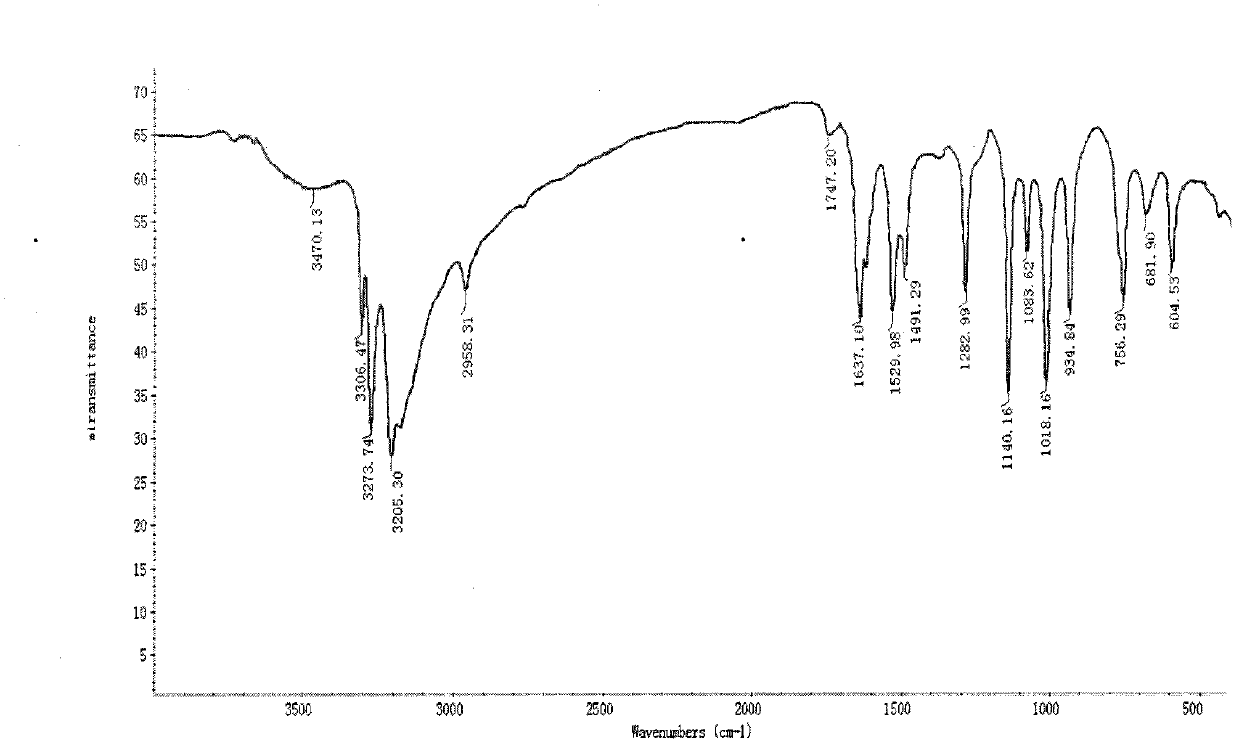
[0046] Infrared spectrum shows: the infrared spectrum of chitosan derivative 2 ( image 3 ) and the infrared spectrum of acetylated chitosan ( figure 1 ) compared to 3470.13, 3306.47, 3273.74cm -1 The absorption peak that appears is the characteristic absorption peak of NH, 1637.10cm -1 It is the characteristic absorption peak of C=N, 1529.98cm -1 It is the characteristic absorption of benzene ring, ...
Embodiment 3
[0047] Example 3 Preparation of Derivative 3
[0048] Add 1.0 g of acetylated chitosan with a molecular weight of 230,000 and 1.77 g of o-fluoroanilinothiourea to 50 mL of N,N-dimethylformamide, add 0.3 mL of acetic acid dropwise under stirring, and reflux for 10 hours , cooled to room temperature, the reactant was suction-filtered, washed with absolute ethanol, and dried at 60°C to obtain a yellow powder, namely acetylated chitosan condensed o-fluorophenylthiosemicarbazide, ie derivative 3, see Table 1 for the structural formula.
[0049] Infrared spectrum shows: the infrared spectrum of chitosan derivative 3 ( Figure 4 ) and the infrared spectrum of acetylated chitosan ( figure 1 ) compared at 3443.64, 3021.16cm -1 The absorption peak that appears is the characteristic absorption peak of NH, 1655.54cm -1 It is the characteristic absorption peak of C=N, 1539.50, 1515.70cm -1 It is the characteristic absorption of benzene ring, 1047.19cm -1 It is the characteristic absor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com