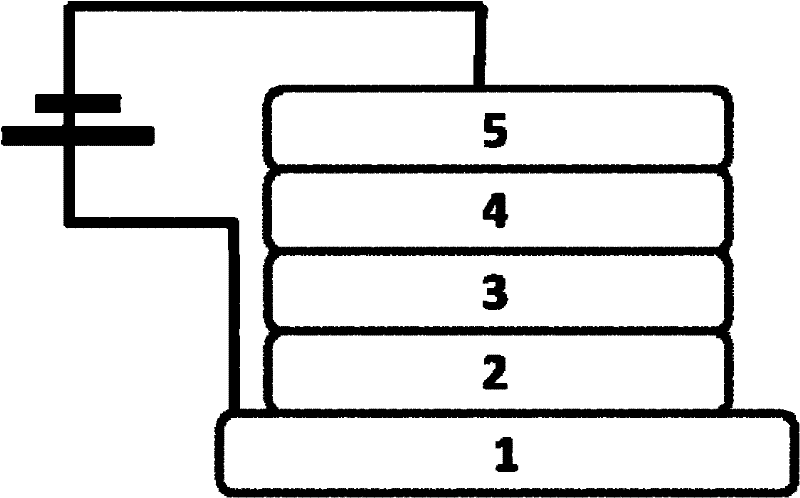
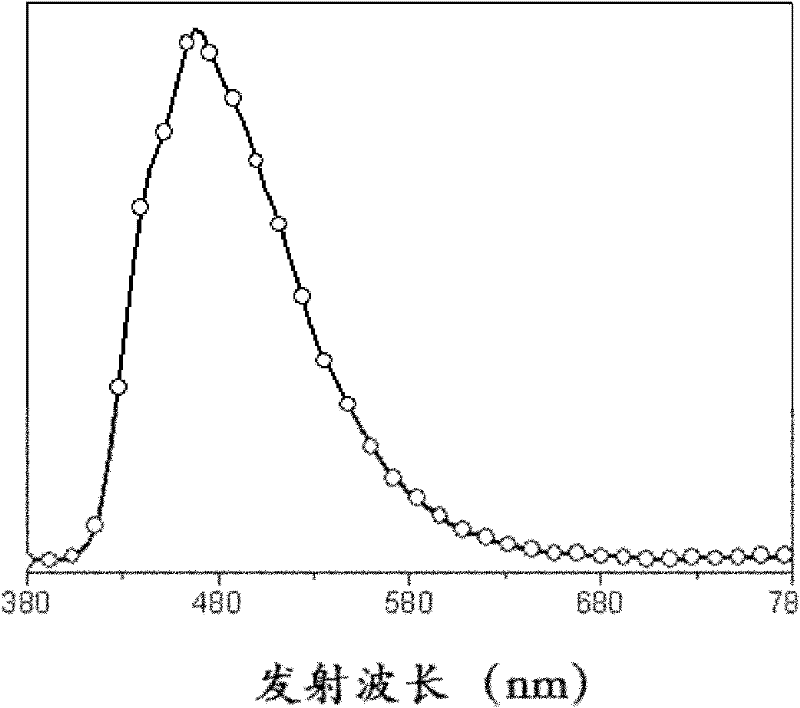
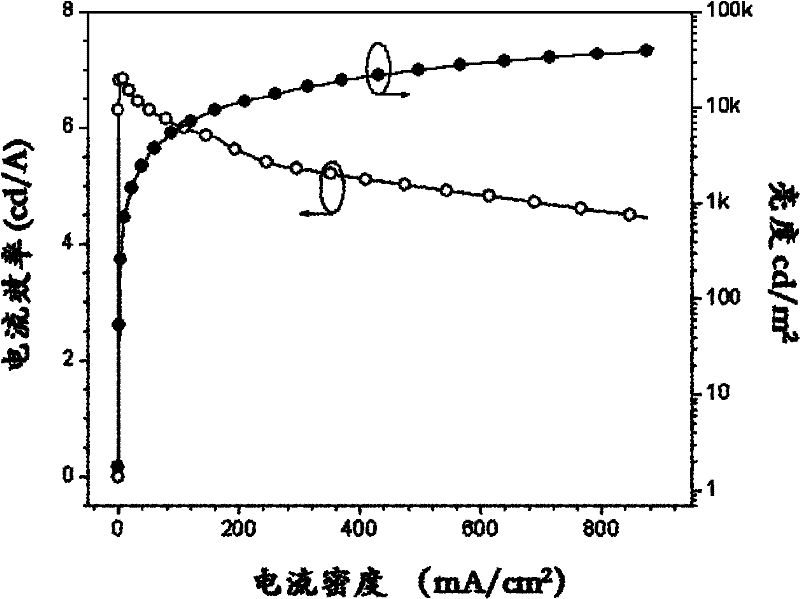
9,10-phenanthroimidazole derivatives and use thereof as electroluminescent materials
A technology of phenanthroimidazole and derivatives, applied in the field of organic electroluminescence, can solve the problems of poor film-forming performance, easy crystallization, low luminous efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: 1,2-diphenyl substituted 9,10-phenanthroimidazole
[0051]
[0052] Heat an appropriate amount of phenanthrenequinone (2.0g), benzaldehyde (0.5ml), aniline (0.5ml) and ammonium acetate (8.0g) in 120ml of glacial acetic acid to reflux at 140°C for two hours, stop heating, and cool to room temperature Filtration afforded a white solid which was taken over SiO 2 Column separation, developing solvent is CH 2 Cl 2 , and purified to obtain the target product with a yield of 90.5%.
[0053] 1 H NMR (500MHz, DMSO, ppm): 8.93(d, J=8.2Hz, 1H), 8.75(d, J=8.5Hz, 1H), 8.69(d, J=7.6Hz, 1H), 7.78(t, J=7.6Hz, 7.0Hz, 1H), 7.73-7.65(m, 6H), 7.58(dd, J=7.9Hz, 2.1Hz, 2H), 7.55(t, J=7.0Hz, 7.3Hz, 1H), 7.39-7.30 (m, 4H), 7.08 (d, J=8.2Hz, 1H). Mass spectrometry data (C 27 h 18 N 2 ) theoretical value: 370.5; measured value: 370.2. Elemental analysis (C 27 h 18 N 2 ) Theoretical: C, 87.54; H, 4.90; N, 7.56. Found: C, 87.50; H, 4.92; N, 7.55.
[0054] It showed that...
Embodiment 2
[0055] Example 2: 4,4-bis-(1-phenyl-9,10-phenanthroimidazole)biphenyl
[0056]
[0057] Heat appropriate amount of phenanthrenequinone (2.0g), 4,4-disubstituted biphenyl dialdehyde (1.0g), aniline (0.5ml) and ammonium acetate (8.0g) in 120ml of glacial acetic acid to reflux at 140°C for 2 hours Afterwards, stop heating, filter after cooling to room temperature, obtain yellow-green solid, adopt SiO 2 Column separation, developing solvent is CH 2 Cl 2 , and purified to obtain the target product with a yield of 80.5%.
[0058] 1 H NMR (500MHz, DMSO, ppm): 8.94 (d, J = 8.5Hz, 2H), 8.89 (d, J = 8.2Hz, 2H), 8.72 (d, J = 8.2Hz, 2H), 7.80-7.65 ( m, 22H), 7.57 (t, J = 7.9Hz, 7.6Hz, 2H), 7.35 (t, J = 7.3Hz, 7.3Hz, 2H), 7.09 (d, J = 8.2Hz, 2H). Mass spectrometry data (C 54 h 34 N 4 ) theoretical value: 738.28; measured value: 739.0. Elemental analysis (C 54 h 34 N 4 ) Theoretical values: C, 87.78; H, 4.64; N, 7.58. Found: C, 87.80; H, 4.62; N, 7.57.
[0059] It showed th...
Embodiment 3
[0060] Example 3: 1,3,5-triphenyl-(1-phenyl-9,10-phenanthroimidazole)benzene
[0061]
[0062] Heat appropriate amount of phenanthrenequinone (2.0g), 1,3,5-triphenyl substituted trialdehyde (0.6g), aniline (1ml) and ammonium acetate (10g) in 120ml of glacial acetic acid to 140°C and reflux for two hours , stop heating, filter after cooling to room temperature, obtain a yellow-green solid, use SiO 2 Column separation, developing solvent is CH 2 Cl 2 , and purified to obtain the target product with a yield of 76.5%.
[0063] 1 H NMR (500MHz, DMSO, ppm): 8.93(d, J=8.8Hz, 3H), 8.88(d, J=7.3Hz, 3H), 8.72(d, J=7.6Hz, 3H), 7.98(s, 3H), 7.93(d, J=8.2Hz, 6H), 7.80-7.66(m, 27H), 7.57(t, J=7.9Hz, 7.6Hz, 3H), 7.35(t, J=8.2Hz, 7.3Hz , 3H), 7.09 (d, J = 7.9 Hz, 3H). Mass spectrometry data (C 87 h 54 N 6 ) Theoretical value: 1182.44; measured value: 1183.50. Elemental analysis (C 87 h 54 N 6 ) Theoretical values: C, 88.30; H, 4.60; N, 7.10. Found: C, 88.26; H, 4.60; N, 7.08....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum lumen efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com