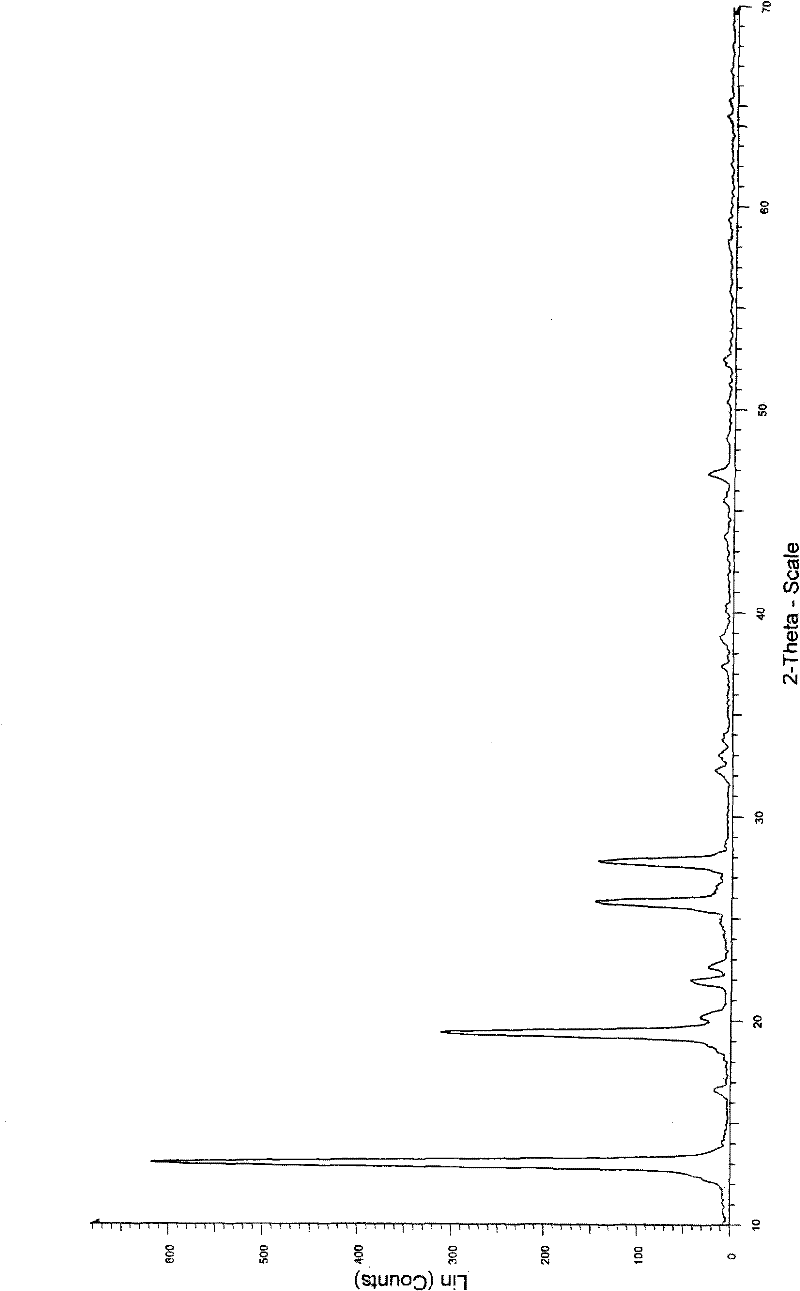
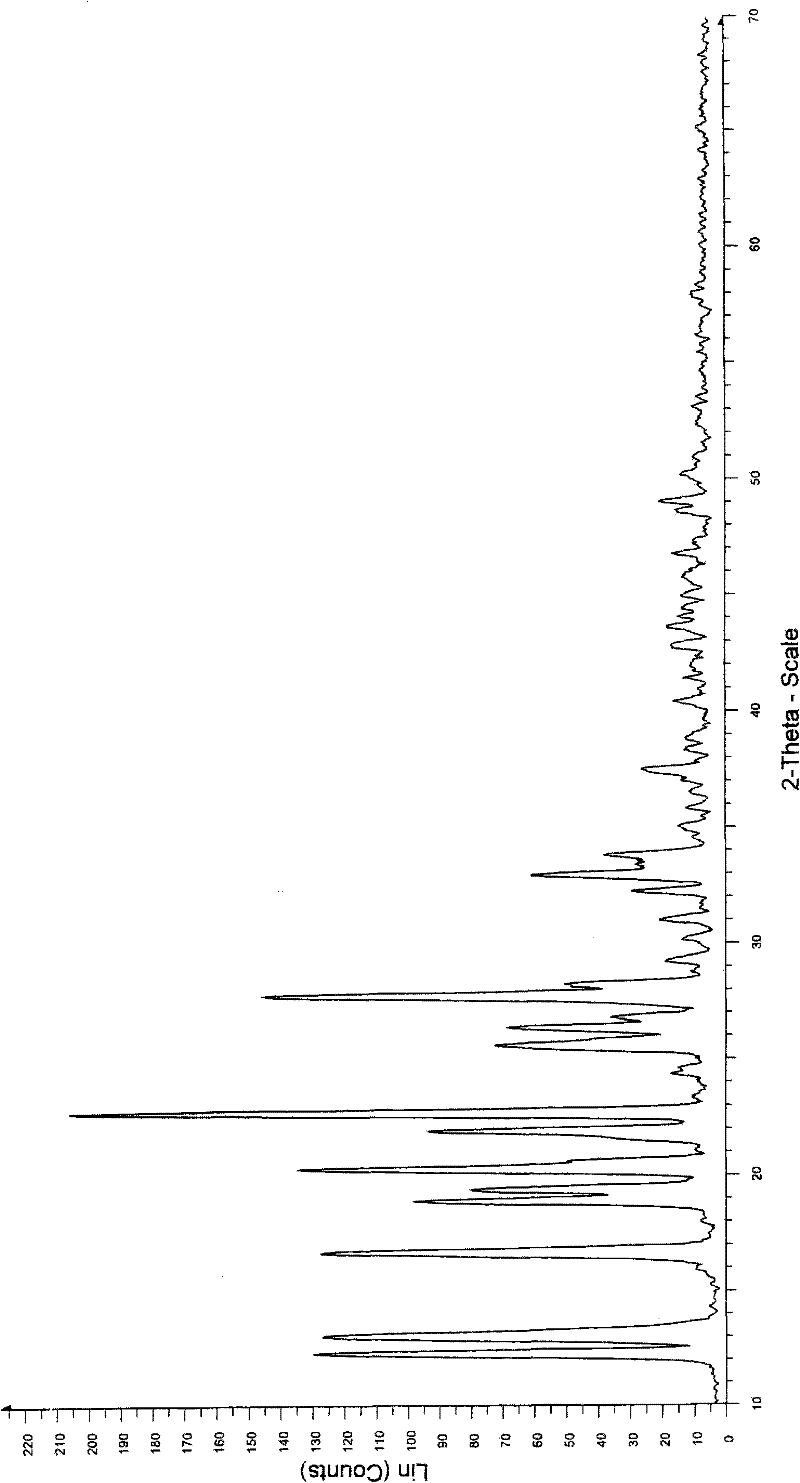
Crystal form of Dimethylamino Arglabin hydrochloride
A technology of crystallization of dimethylamino and hydrochloride, applied in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as slow dissolution, difficult preparation of preparations, and easy generation of static electricity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of dimethylaminoagrabine hydrochloride of form I (with reference to ZL97181524.0 method)
[0040] Add 1 g (3.4 mmol) of dimethylaminoagrabine to 20 ml of absolute ethanol, stir and dissolve at 10°C, then bubble hydrogen chloride gas into the solution to make the pH of the solution reach 5.0-5.5, evaporate the ethanol, add 20ml of ethyl acetate was stirred for crystallization, and suction filtered. The resulting product was dissolved in 20ml of chloroform, the chloroform was evaporated, and 20ml of ethyl acetate was added under vigorous stirring, and the resulting precipitate was dried in a vacuum oven at 25°C for 5h to obtain 0.5g (44.4%) of dimethylaminoagrabine in form I Hydrochloride, the HPLC purity is 95.1%.
Embodiment 2
[0041] Embodiment 2: Preparation of dimethylaminoagrabine hydrochloride of form I
[0042] Add 1 g (3.4 mmol) of dimethylaminoagrabine into 40 ml of ethyl acetate, stir to dissolve at 10°C, add 0.4 ml of 35% ethanol hydrochloric acid solution dropwise, stir for 30 min, precipitate crystals, filter with suction, and obtain precipitates in a 25°C After drying in a vacuum oven for 5 h, 0.65 g (57.8%) of form I dimethylaminoagrabine hydrochloride was obtained, with an HPLC purity of 99.6%.
Embodiment 3
[0043] Example 3: Preparation of Dimethylaminoagrabine Hydrochloride of Form II
[0044] Add 2 g (6.1 mmol) of dimethylaminoagrabine hydrochloride in form I into 20 ml of acetone, heat to dissolve, crystallize at 0±10° C., and filter with suction. After drying in a vacuum oven at 22° C. for 5 h, 1.0 g (50%) of dimethylaminoagrabine hydrochloride in form II was obtained, with an HPLC purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com