Analysis method for colistimethate sodium
A technology of polymyxin and sodium methanesulfonate, which is applied in the analysis of materials, material separation, measuring devices, etc., can solve the problems of unproven colistin, etc., and achieve the effect of convenient standardized operation and real and effective results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of polymyxin E sulfate
[0033] Weigh an appropriate amount of industrial-grade polymyxin E sulfate, add water to dissolve, and make a solution containing 100 mg per 1 ml, and separate it on a chromatographic column. The chromatographic conditions are as follows:
[0034] Chromatography packing: polystyrene / divinylbenzene as the skeleton and bonded phenyl, model NM-100, produced by Suzhou Nano Microbe Technology Co., Ltd.
[0035] Column specification: 45×400mm,
[0036] Column volume (CV): 635.9ml
[0037] Flow rate: 30.0ml / min
[0038] Sample volume: 500ml
[0039] Elution conditions:
[0040] Wash the mobile phase: first wash 10CV with a dilute sulfuric acid solution containing 8% ethanol (adjust the pH of the solution to 2.4 with 0.05mol / L sulfuric acid).
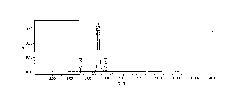
[0041]Eluent: then use 30% ethanol dilute sulfuric acid solution (use 0.05mol / L sulfuric acid to adjust the pH value of the solution to 2.4.) to elute 10CV. The eluate was coll...
Embodiment 2
[0042] Embodiment 2: polymyxin E sulfate 1 and E 2 preparation of
[0043] Weigh an appropriate amount of industrial-grade polymyxin E sulfate, add water to dissolve, and make a solution containing 100 mg per 1 ml, and separate it on a chromatographic column. The chromatographic conditions are as follows:
[0044] Column: C 18 SunFire Prep OBD (10μm); 19×150mm, purchased from WATERS, USA
[0045] Column volume (CV): 42.5ml
[0046] Flow rate: 10.0ml / min
[0047] Elution conditions: 30CV isocratic elution with dilute sulfuric acid solution containing 30% ethanol (use 0.05mol / L sulfuric acid to adjust the pH value of the solution to 2.3.).
[0048] Sample volume: 50ml
[0049] Collection: 10ml / tube
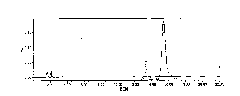
[0050] Analysis and processing of collected samples: analyzed by analytical HPLC (chromatographic conditions refer to the purity determination of polymyxin E in European Pharmacopoeia 5.0), and the combined purity of polymyxin E is above 95%. 1 and E 2 The components are t...
Embodiment 3
[0052] Embodiment 3: the preparation of polymyxin E sodium methanesulfonate
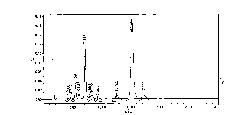
[0053] Weigh 10.0 g of the polymyxin E sulfate prepared by the method in Example 1, add water to dissolve and set the volume to 50 ml, then add 6.2 ml of formaldehyde solution, stir and adjust the pH to 7.0 with 10 mol / L NaOH solution, after maintaining the reaction for 30 min, 28 ml of 40% sodium bisulfite solution was added thereto, and then the pH was adjusted to 7 with NaOH solution, and the reaction was stopped after stirring for 10 h. Get this reaction solution, ultrafiltration to about 20ml with a molecular weight cut-off of 3000 ultrafiltration membrane (the product of U.S. MILLIPORE company, is a cellulose acetate membrane), then add water to about 100ml, then ultrafiltration and concentration to 20ml, so repeated for 5 times, about 15ml of ultrafiltration retentate was obtained. The temperature was controlled at 20-25°C throughout the reaction and ultrafiltration period. The ultrafiltrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com