10-hydroxycamptothecine invisible nano-particle sustained release preparation and preparation method thereof
A technology of hydroxycamptothecin and sustained-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., which can solve problems such as increased disease sensitivity and MPS self-injury, and achieve Effects of reduced adsorption, high drug loading rate, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 10-hydroxycamptothecin stealth nanoparticle sustained-release preparation: Weigh 40 mg of 10-hydroxycamptothecin bulk drug, polylactic acid grafted with polyethylene glycol monomethyl ether with a molecular weight of 10,000 Daltons (mPEG-PLA) 400mg, put it in a beaker and dissolve it with 10ml DMF (N,N-dimethylformamide); 1, 4, and 8 hours to replace the water phase in the bathtub to achieve complete dialysis; freeze-dry the resulting suspension to obtain 10-hydroxycamptothecin invisible nano drug-loaded microspheres, which are sterilized and subpackaged.
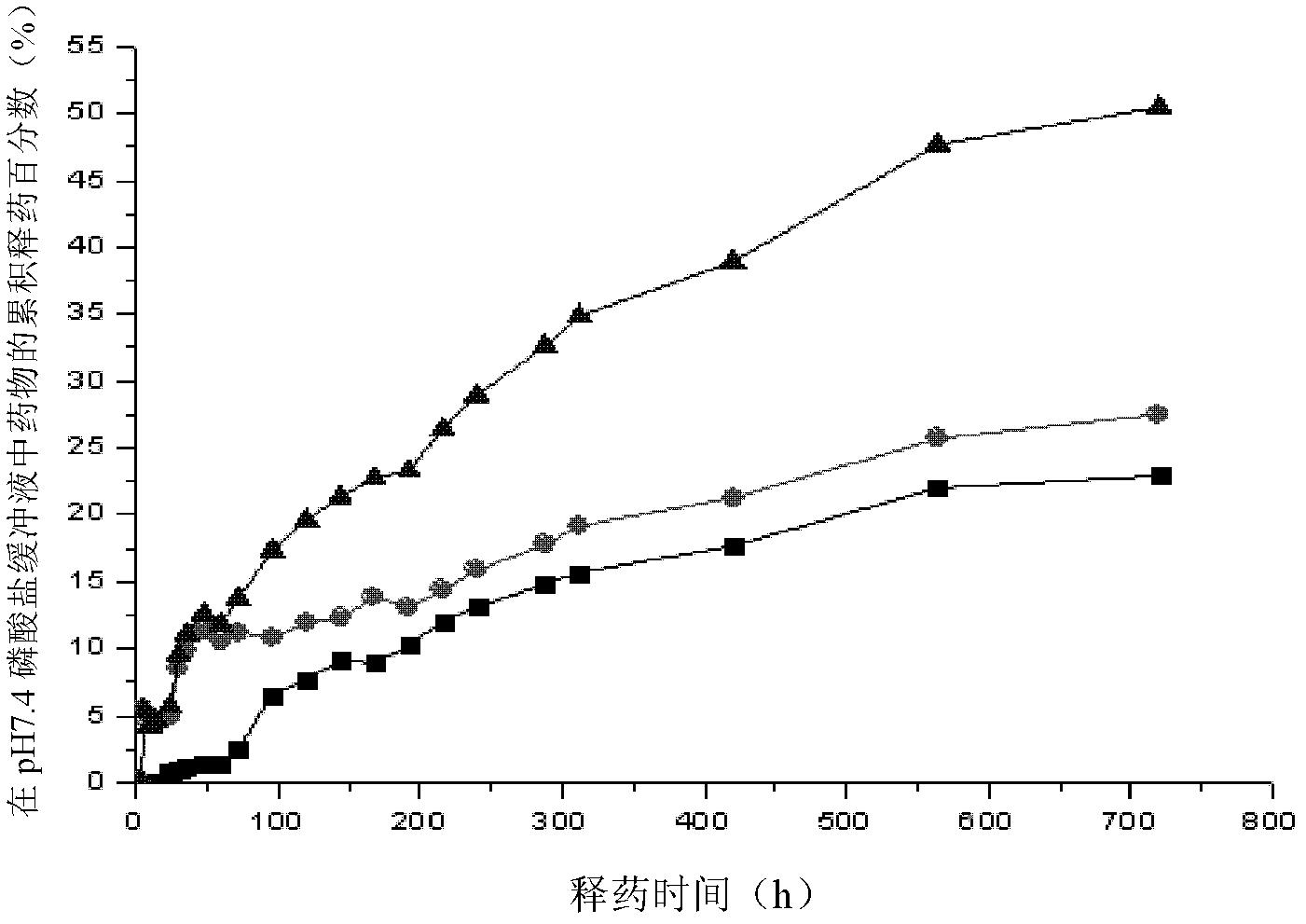
[0032] Utilize this method to prepare microsphere process to cause carrier material loss substantially; Simultaneously 10-hydroxycamptothecin itself is insoluble in water, and loss is little; From figure 1 The scanning electron microscope image shows that the particle size of the drug-loaded microspheres is round, the average particle size is 210nm, and the drug-loading capacity is 7.1%. f...
Embodiment 2
[0033] Example 2: Preparation of 10-Hydroxycamptothecin Stealth Nanoparticle Sustained Release Preparation: Weigh 40 mg of 10-Hydroxycamptothecin bulk drug, polylactic acid grafted with molecular weight 35000 Dalton polyethylene glycol monomethyl ether (mPEG-PLA) 400mg, put it in a beaker and dissolve it with 10ml DMF (N,N-dimethylformamide); 1, 4, and 8 hours to replace the water phase in the bathtub to achieve complete dialysis; freeze-dry the obtained suspension, sterilize and subpackage. The particle size of the drug-loaded microspheres is rounded ( Figure 4 ), the average particle size is 230nm, the drug loading capacity is 7.6%, and the average drug release time in vitro can reach more than 30 days.
Embodiment 3
[0034] Embodiment 3: Preparation of 10-hydroxycamptothecin invisible nanoparticle slow-release preparation: Weigh 40 mg of 10-hydroxycamptothecin bulk drug, and use molecular weight of 10000 Dalton polyethylene glycol monomethyl ether grafted poly( Lactic acid-glycolic acid) copolymer (mPEG-PLGA) 400mg, put in the beaker and dissolve with 10ml DMF (N, N-dimethylformamide); The above-mentioned solution is transferred to the dialysis bag of minimum permeability 8000 Dalton, Put it in a water bathtub, and replace the water phase in the bathtub every 1, 4, and 8 hours to complete the dialysis; freeze-dry the obtained suspension, and sterilize it for packaging. The particle size of the drug-loaded microspheres is rounded ( Figure 5 ), the average particle size is 224nm, the drug loading capacity is 7.3%, and the average drug release time in vitro can reach more than 30 days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com