A kind of method for preparing type I clopidogrel bisulfate
A technology of clopidogrel bisulfate and clopidogrel free base, which is applied in organic chemistry and other fields, and can solve problems such as cumbersome operation, unguaranteed crystal purity, and high residual solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
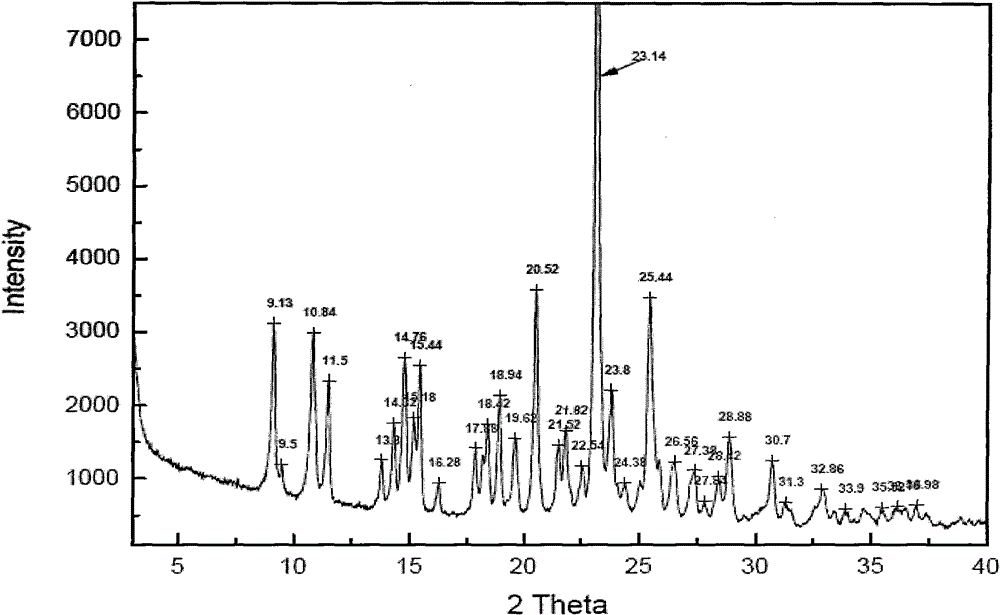
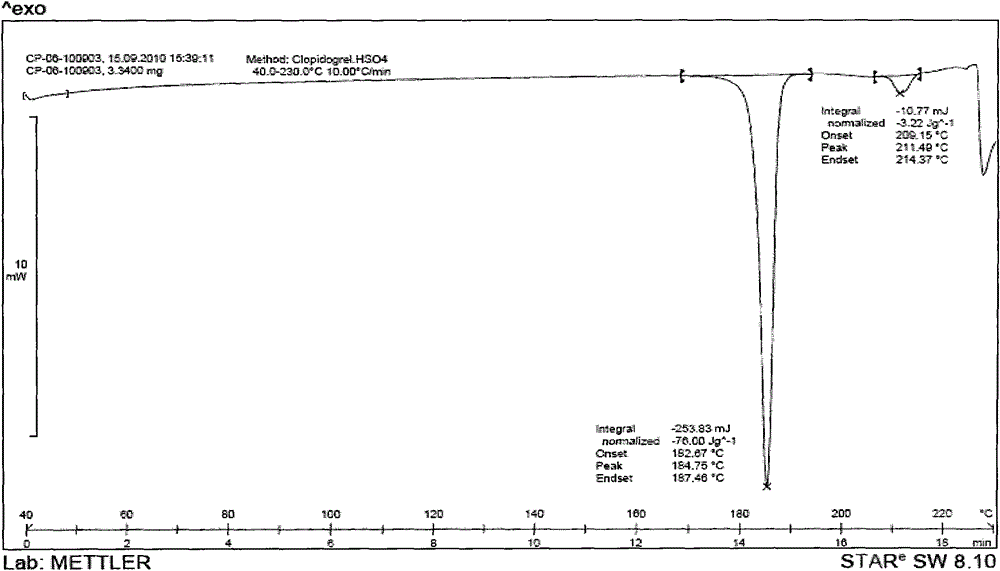
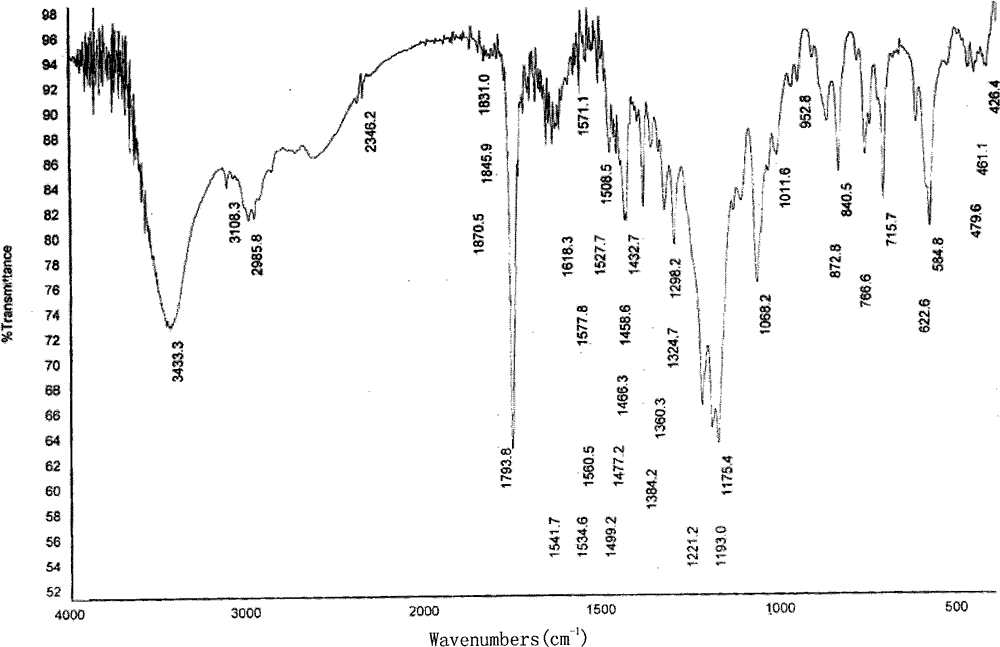
Image
Examples
Embodiment 1
[0046] Draw 3.83kg of clopidogrel free base and 45.0kg of methyl isobutyl ketone into a dry reaction kettle, stir to dissolve, cool to below -5°C, maintain T=-10±5°C, slowly drop to configure After dropping the good diluted methyl isobutyl ketone sulfate solution, slowly warm up to T=30±5°C, keep stirring for 2 to 4 hours, filter, and wash the filter cake with a small amount of methyl isobutyl ketone. Vacuum drying for 8 hours yielded 4.5 kg of white crystals.
[0047] Yield: 90%
Embodiment 2
[0049] Put 4.0 kg of the above clopidogrel bisulfate crystal form Form I into 20 L of acetone, cool to -10°C, stir for 1 to 2 hours, filter, wash the filter cake with a small amount of frozen acetone, and dry it in vacuum at 45°C for 8 hours to obtain Product: 3.6 kg.
[0050] Yield: 90% HPLC: 99.8% Optical purity: 99.85%
Embodiment 3
[0052] Put 4.0 kg of the above-mentioned clopidogrel bisulfate crystal form Form I into 12 L of butanone, cool to -10°C, stir for 1 to 2 hours, filter, wash the filter cake with a small amount of frozen acetone, and dry it in vacuum at 45°C for 8 hours. Product obtained: 3.68 kg.
[0053] Yield: 92% HPLC: 99.7% Optical purity: 99.85%
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com