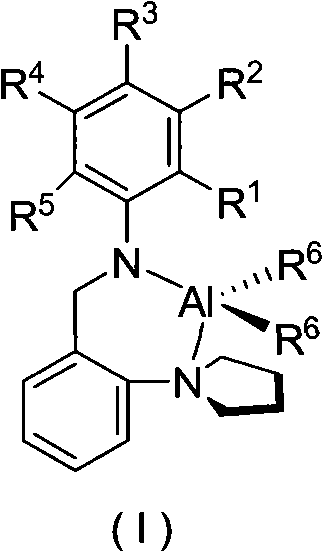
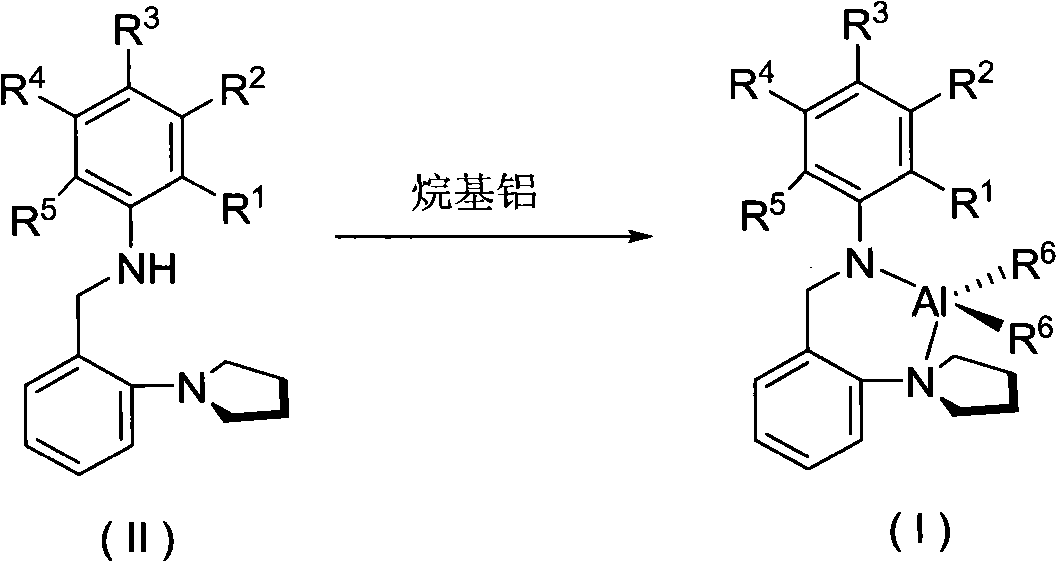
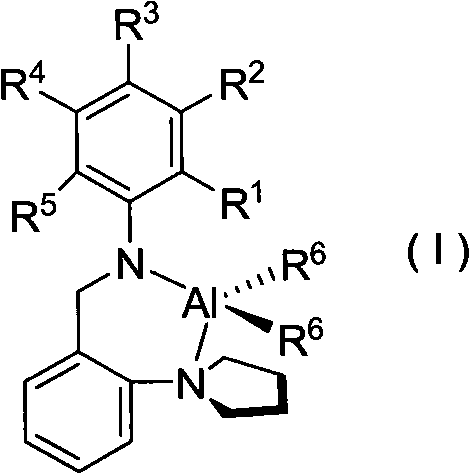
Pyrrolidyl amino bidentate ligand aluminum complex and preparation method and application thereof
A technology of pyrrolidinylamine and bidentate ligand is applied to the application field of lactone ring-opening polymerization, which can solve the difficulty of industrial production, ε-caprolactone does not have any catalytic activity, and the catalytic activity and selectivity are relatively affected. It can achieve the effect of highly controllable molecular weight, stable properties and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of complex C1
[0034] To a solution of 2,6-dimethyl-N-(2-pyrrolidinyl-benzyl)-aniline (10 mL, 4 mmol) in n-hexane at room temperature, slowly add a solution of trimethylaluminum in toluene (2 mL, 4 mmol) , stirred at room temperature for 20h. The solvent was removed by filtration, and the remaining solid was crystallized from a mixed solution of dichloromethane and n-hexane to obtain 0.457 g of colorless crystals (yield: 34%).
[0035]
[0036] 1 H NMR (400MHz, CDCl 3 ): δ7.20-7.12(t, J=6.7Hz, 1H, Ar-H), 7.04(d, J=8.3Hz, 1H, Ar-H), 6.90(m, 3H, Ar-H), 6.83 (d, J=1.6Hz, 1H, Ar-H), 6.79-6.70 (d, J=7.6Hz, 1H, Ar-H), 4.44 (br, 2H, phenyl-CH 2 ), 3.18(br, 4H, N(CH 2 )), 2.22 (br, 6H, phenyl-CH 3 ), 1.98 (br, 4H, CH 2 ), -0.94(s, 6H, Al-CH 3 ). 13 C NMR (100MHz, CDCl 3 ): δ153.16(Ar-C), 149.99(Ar-C), 132.02(Ar-C), 132.22(Ar-C), 131.83(Ar-C), 130.24(Ar-C), 129.84(Ar-C C), 129.49(Ar-C), 128.50(Ar-C), 125.27(Ar-C), 125.18(Ar-C), 123.84(Ar-C), 122.10(Ar-C), ...
Embodiment 2
[0038] Synthesis of complex C2
[0039] To a solution of 4-chloro-N-(2-pyrrolidinyl-benzyl)-aniline (10 mL, 4 mmol) in n-hexane at room temperature, trimethylaluminum toluene solution (2 mL, 4 mmol) was slowly added, and stirred at room temperature for 20 h. The solvent was removed by filtration, and the remaining solid was crystallized from a mixed solution of dichloromethane and n-hexane to obtain 0.561 g of colorless crystals (yield: 41%).
[0040]
[0041] 1 H NMR (400MHz, CDCl 3 ): δ7.27 (t, J=5.3, 1H, Ar-H), 7.21 (d, J=7.1, 1H, Ar-H), 7.16 (t, J=4.7, 1H, Ar-H), 7.09 (d, J=1.2Hz, 1H, Ar-H), 7.03-7.00 (d, J=7.6, 2H, Ar-H), 6.54-6.52 (d, J=7.4, 2H, Ar-H), 4.31 (s, 2H, phenyl-CH 2 ), 3.44(m, 4H, N(CH 2 )), 1.97(m, 4H, CH 2 ), -0.98(s, 6H, Al-CH 3 ). 13 C NMR (100MHz, CDCl 3 ): δ152.88(Ar-C), 144.80(Ar-C), 134.13(Ar-C), 132.11(Ar-C), 128.46(Ar-C), 128.12(Ar-C), 127.22(Ar-C C), 119.05(Ar-C), 119.00(Ar-C), 115.34(Ar-C), 54.16(phenyl-CH 2 ), 53.62 (N (CH 2 )), 23....
Embodiment 3
[0043] Synthesis of complex C3
[0044] To a solution of 4-isopropyl-N-(2-pyrrolidinyl-benzyl)-aniline (10 mL, 4 mmol) in n-hexane at room temperature, slowly add trimethylaluminum toluene solution (2 mL, 4 mmol), and stir at room temperature 20h. The solvent was removed by filtration, and the remaining solid was crystallized from a mixed solution of dichloromethane and n-hexane to obtain 0.462 g of colorless crystals (yield: 33%).
[0045]
[0046] 1 H NMR (400MHz, CDCl 3 ): δ7.28-7.25(d, J=7.8Hz, 1H, Ar-H), 7.20-7.18(t, J=5.3Hz, 1H, Ar-H), 7.16-7.12(t, J=4.7Hz , 1H, Ar-H), 7.08 (d, J=6.8Hz, 1H, Ar-H), 6.99-6.96 (d, J=8.2Hz, 2H, Ar-H), 6.59 (d, J=6.5, 2H, Ar-H), 4.37(s, 2H, phenyl-CH 2 ), 3.44(m, 4H, N(CH 2 )), 2.72(h, J=1.1Hz, 1H, CH(CH 3 ) 2 ), 1.97 (m, 4H, CH 2 ), 1.14(d, J=6.9Hz, 6H, CH(CH 3 ) 2 ), -0.97(s, 6H, Al-CH 3 ). 13 C NMR (100MHz, CDCl 3 ): δ152.20(Ar-C), 144.92(Ar-C), 134.74(Ar-C), 134.70(Ar-C), 132.02(Ar-C), 127.80(Ar-C), 127.06(Ar-C C), 126.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com