Genetic engineering preparation method of cytidine triphosphate
A technology of cytidine triphosphate and genetically engineered bacteria, which is applied in the field of preparation of cytidine triphosphate, can solve problems such as long conversion time, impact on conversion rate, and increase in clinical dosage, and achieve easy control of reaction conditions, rapid reaction, and high conversion rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
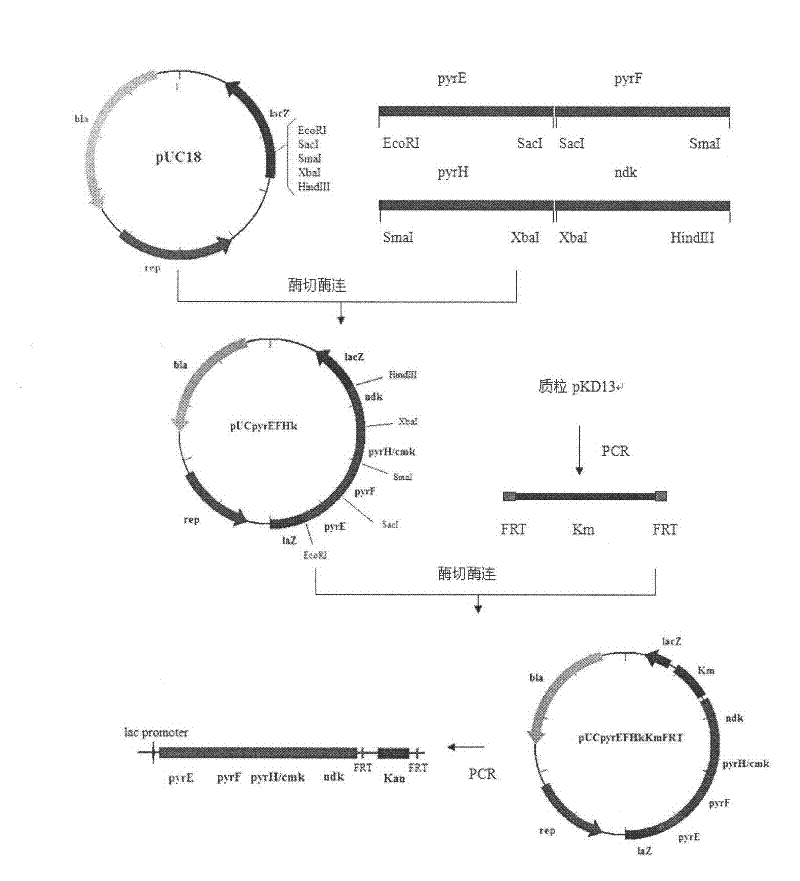
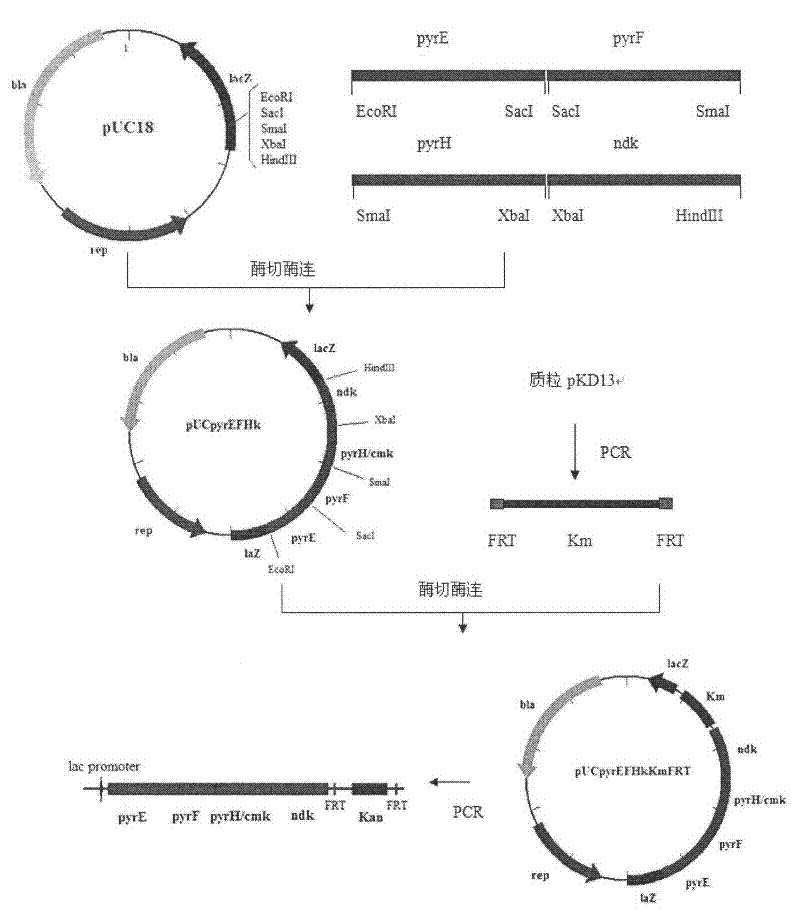
[0023] Embodiment 1, construction of Escherichia coli with pyrE, pyrF, pyrH, ndk operator on chromosome
[0024] 1.1. Amplification of pyrE, pyrF, pyrH, ndk genes and construction of vectors
[0025] The primers for pyrE gene, pyrF gene, pyrH gene, ndk gene and pyrG gene were designed according to the whole genome sequence of Escherichia coli (genebank accession number: ECK3632).
[0026] The genome of Escherichia coli K12MG1655 was extracted.
[0027] Then, the pyrE, pyrF, pyrH, and ndk genes were respectively amplified using the Escherichia coli K12MG1655 genome as a template. After sequencing, the pyrE fragment was connected to the plasmid pUC18 through the EcoRI and SacI restriction sites to obtain the plasmid pUCpyrE; The pyrF fragment was connected to the plasmid pUCpyrE by the SmaI site to obtain the plasmid pUC-pyrEF; then the pyrH fragment was connected to pUC-pyrEF via the SmaI and XbaI sites to obtain the plasmid pUCpyrEFH; finally the ndk fragment was connected by...
Embodiment 2
[0042] Embodiment 2, pyrG gene expression vector construction
[0043] According to the known nucleic acid sequence of the pyrG gene of Escherichia coli K12MG1655, the full-length pyrG gene is amplified by using the chromosome of Escherichia coli as a template.
[0044] The genomic DNA of Escherichia coli strain DH5α preserved in our laboratory was extracted by conventional bacterial DNA extraction method.
[0045] The pfu enzyme with good fidelity was used for amplification, and after adding A tail, it was connected to pUC19-T vector, and the positive clones were picked and sent to the sequencing company, and the sequence confirmed by sequencing was used in the next step.
[0046] The pyrG gene connected to the pUC19-T vector was digested from the vector, the restriction enzymes used were Sma I and BamH I, and the vector pUC18 was digested with restriction enzymes Nru I and BamH I, After recovering the digested product of the vector, perform an enzyme ligation reaction with ...
Embodiment 3
[0047] Embodiment 3, the construction of the genetic engineering bacterium that produces cytidine triphosphate
[0048] Prepare the recombinant K1-E obtained in Example 1 as a competent cell, and then transform the plasmid pUCG constructed in Example 2 into K1-E, and name it K1-E / pUCG, namely Genetically engineered bacteria capable of catalyzing the synthesis of cytidine triphosphate, such as orotate and ammonium chloride, are stored in a -80°C refrigerator with glycerin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com