Sintered lithium complex oxide
A composite oxide and sintered body technology, which is applied in the fields of cobalt compounds, manganese compounds, inorganic chemistry, etc., can solve the problems of unrecorded particle or pore forming agent manufacturing methods, etc., and achieve the effect of excellent high-speed discharge characteristics and stable porous structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
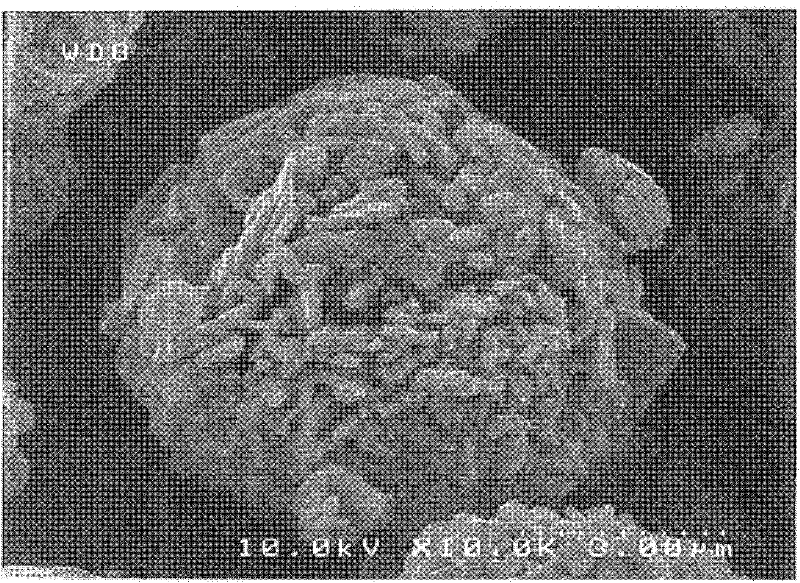
Image
Examples
Embodiment 1
[0153]
[0154] Japanese synthesis of adding 9.6 g of commercially available polymethyl methacrylate particles (MX-150 manufactured by Soken Chemical Co., Ltd., average particle size: 1.5 μm) to polyvinyl alcohol having a quaternary ammonium salt group on the side chain To 32 g of a 4% by weight aqueous solution of Gohsefimer K-210 manufactured by Chemical Corporation, ultrasonic dispersion treatment was performed for 1 minute, and then 168 g of ion-exchanged water was added, and ultrasonic dispersion treatment was performed again for 1 minute. After diluting the obtained polymer emulsion (slurry A) containing surface-modified resin particles (particle A) with ion-exchanged water according to the aforementioned sample conditions, the zeta potential was measured. As a result, it was confirmed that the zeta potential of the particle A was a positive value ( +25mV).
[0155]
[0156] Prepared by adding 225 g of lithium cobaltate particles (manufactured by Honjo Chemical, HLC-...
Embodiment 2
[0164]
[0165] tert-butyl methacrylate (50 g), ion-exchanged water (250 g), a 4% by weight aqueous solution (25 g) of Gohsefimer K-210 manufactured by Nippon Synthetic Chemicals Co., Ltd., emulgen 1135S manufactured by Kao Corporation as a nonionic surfactant- 70 (0.36 g), as an initiator, V-65 (0.15 g) manufactured by Wako Pure Chemical Industries, Ltd. was mixed with an ultrasonic homogenizer (manufactured by NISSEI Corporation, Ultrasonic Generator; US-300T type, probe diameter: 20 mm, electric Flat: 400 μA) was subjected to dispersion treatment for 5 minutes to emulsify it, and then all was added to a 1 L separable flask, and the temperature was raised to 55° C. while stirring under a nitrogen atmosphere, and stirred for 3 hours. Then, the temperature was raised to 65° C. and aged for 1.25 hours. After aging, cool to room temperature to obtain slurry A. The average particle diameter of the particles A contained in this polymer emulsion was 5.0 μm. In addition, the zet...
Embodiment 3
[0173] Except that the maximum calcining temperature in the calcining step was set to 650°C, operations from the preparation of the slurry to the calcining step were performed in the same manner as in Example 1 above to obtain sintered lithium cobaltate particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com