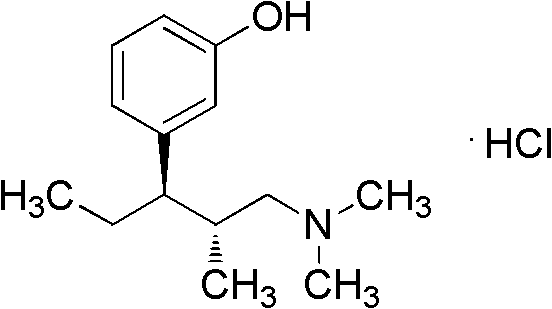
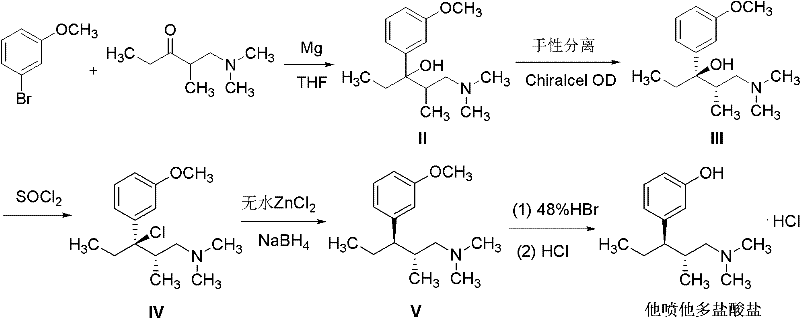
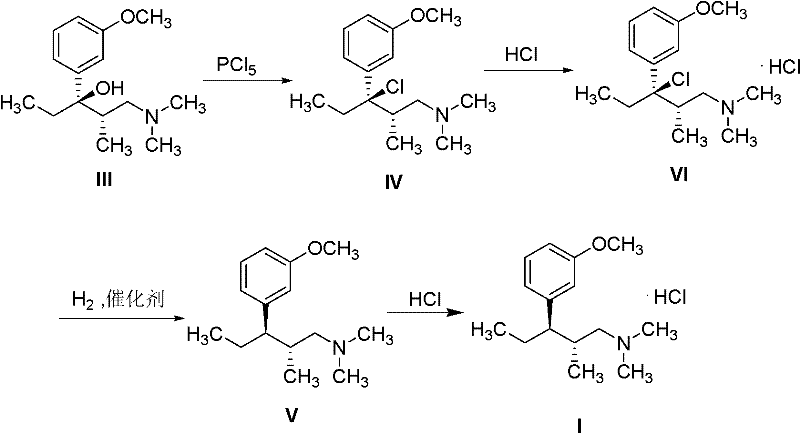
Method for preparing tapentadol intermediate
A technology of intermediates and catalysts, applied in the field of drug synthesis, can solve the problem of large release, and achieve the effects of high purity, simple post-processing, and convenient feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of (2S,3R)-[3-chloro-3-(3-methoxyphenyl)]-N,N,2-trimethylpentylamine hydrochloride (IV)
[0020] Weigh (2S, 3R)-1-(dimethylamino)-3-(3-methoxyphenyl)-2-methylpentan-3-ol III 10.0g (39.8mmol) in a 100ml eggplant-shaped bottle , add 40ml of dichloromethane, add phosphorus pentachloride 9.8g (47.8mmol) in batches under stirring, heat up and reflux for 2h, cool, add 10% sodium hydroxide aqueous solution to adjust the pH to 9-10, filter, and the filtrate is dichloromethane Methane was extracted three times (40ml×3), and the dichloromethane layer was dried over anhydrous magnesium sulfate. After filtration, the filtrate was spin-dried to obtain 8.2 g of yellow oily substance IV, which was dissolved by adding 10 ml of 2-butanone and passing through dry hydrogen chloride gas, cooling to precipitate a white solid VI (6.6 g, 54.2%), melting point 148-149°C. 1 H-NMR (300MHz, CDCL 3 ), δ (ppm): 0.67 (3H, t, J=6.8Hz, CH 3 ), 0.89 (3H, d, J=6.2Hz, CH 3 ), 2.28~2.33 (2H,...
Embodiment 2
[0022] Synthesis of (2R,3R)-3-(3-methoxyphenyl)-N,N,2-trimethylpentylamine hydrochloride (I)
[0023] Weigh IV (6.6g, 21.5mmol) into a 100ml eggplant-shaped bottle, add methanol 50ml, 10% Pd / C (0.6g, 6.1mmol), react with hydrogen at 40-45°C for 6h under normal pressure, filter, evaporate Remove the solvent, add 40ml of ethyl acetate to the residue, adjust the pH to 9-10 by adding 10% ammonia solution, extract with ethyl acetate (30ml×3), combine the organic phases, and dry over anhydrous magnesium sulfate. Filter and evaporate to dryness to obtain 5.9 g of pale yellow oily substance V. After dissolving in ethyl acetate, pass through dry hydrogen chloride gas to form hydrochloride. Filter to obtain white solid I (4.9 g, 84%), melting point 162-164 ° C, 1 H-NMR (300MHz, CDCL 3 ), δ (ppm): 0.70 (3H, t, CH 3 ), 1.01 (3H, d, CH 3 ), 1.57~1.61 (1H, m, CH), 1.75~1.83 (1H, m, 1 / 2CH 2 ), 1.87~1.92 (1H, m, 1 / 2CH 2 ), 2.13~2.17 (2H, m, CH 2 ), 2.29 (6H, s, N (CH 3 ) 2 ), 2.25~2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com