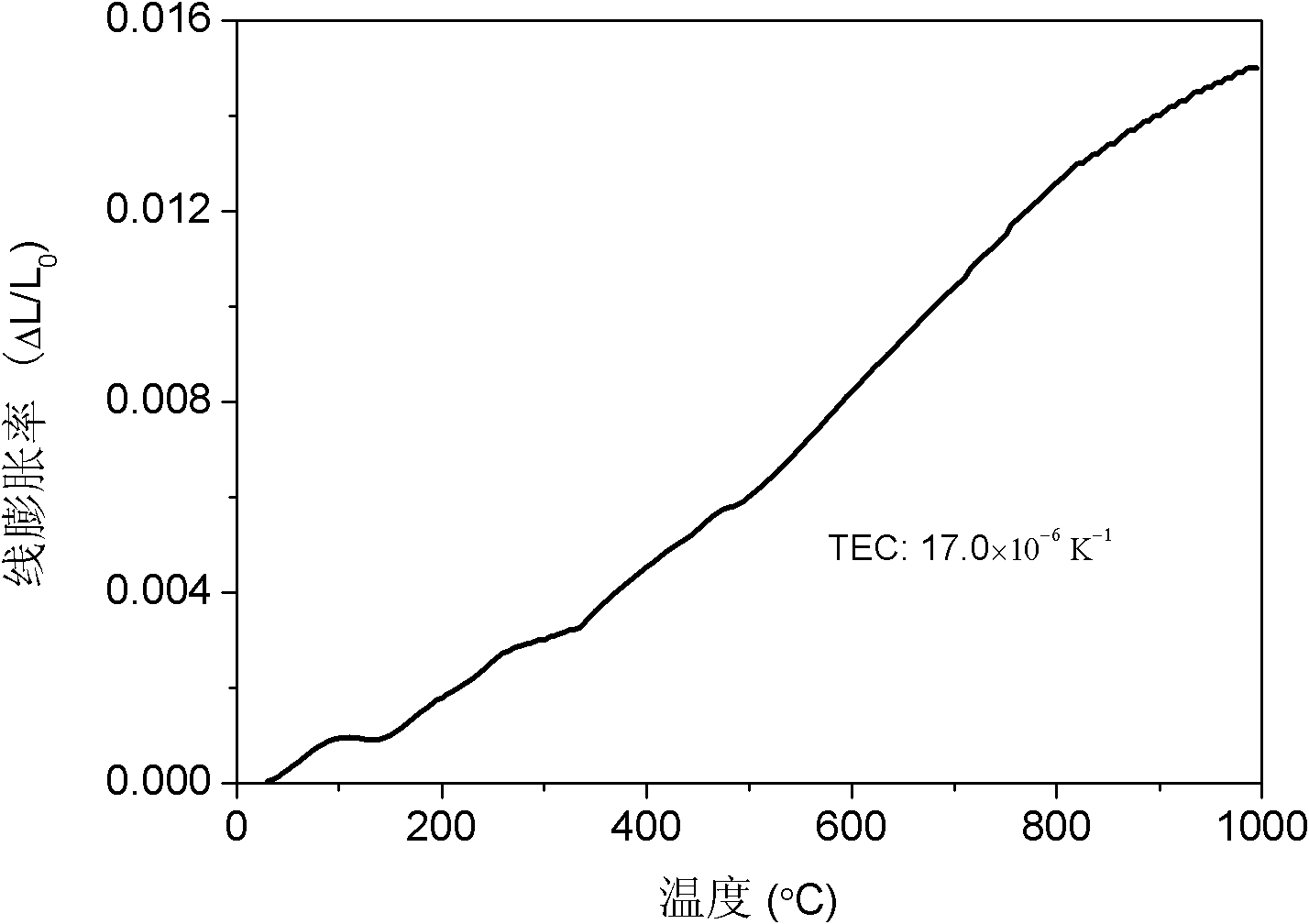
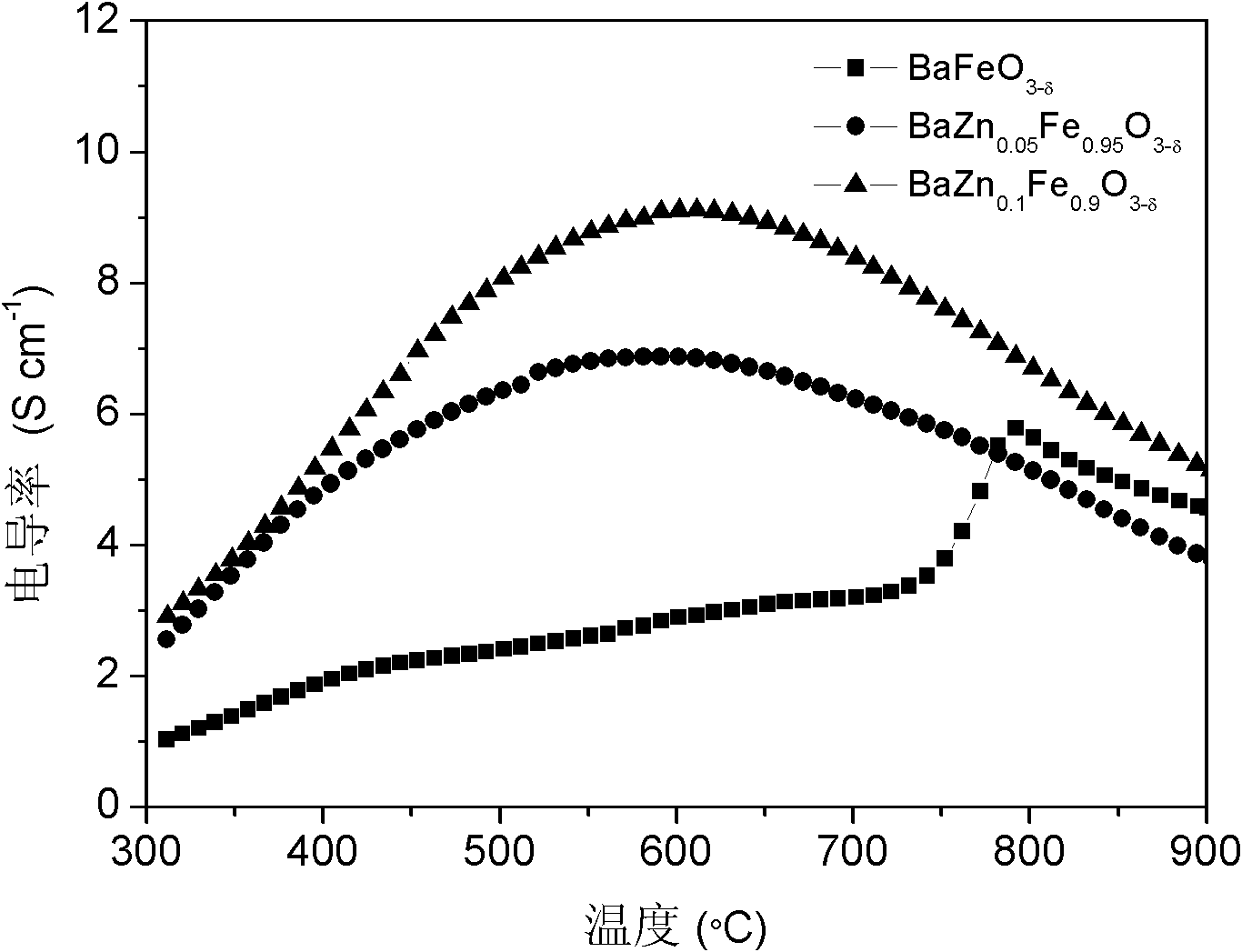
Transition metal element B site-doped BaFeO3-delta-based ABO3 type perovskite fuel cell cathode material and application thereof
A transition metal element, fuel cell cathode technology, applied in the direction of battery electrodes, circuits, electrical components, etc., to achieve the effect of enhancing mixed electrical conductivity, improving performance, and good oxygen catalytic reduction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: BaNb 0.05 Fe 0.95 o 3-δ Preparation of cathode material (solid phase method)
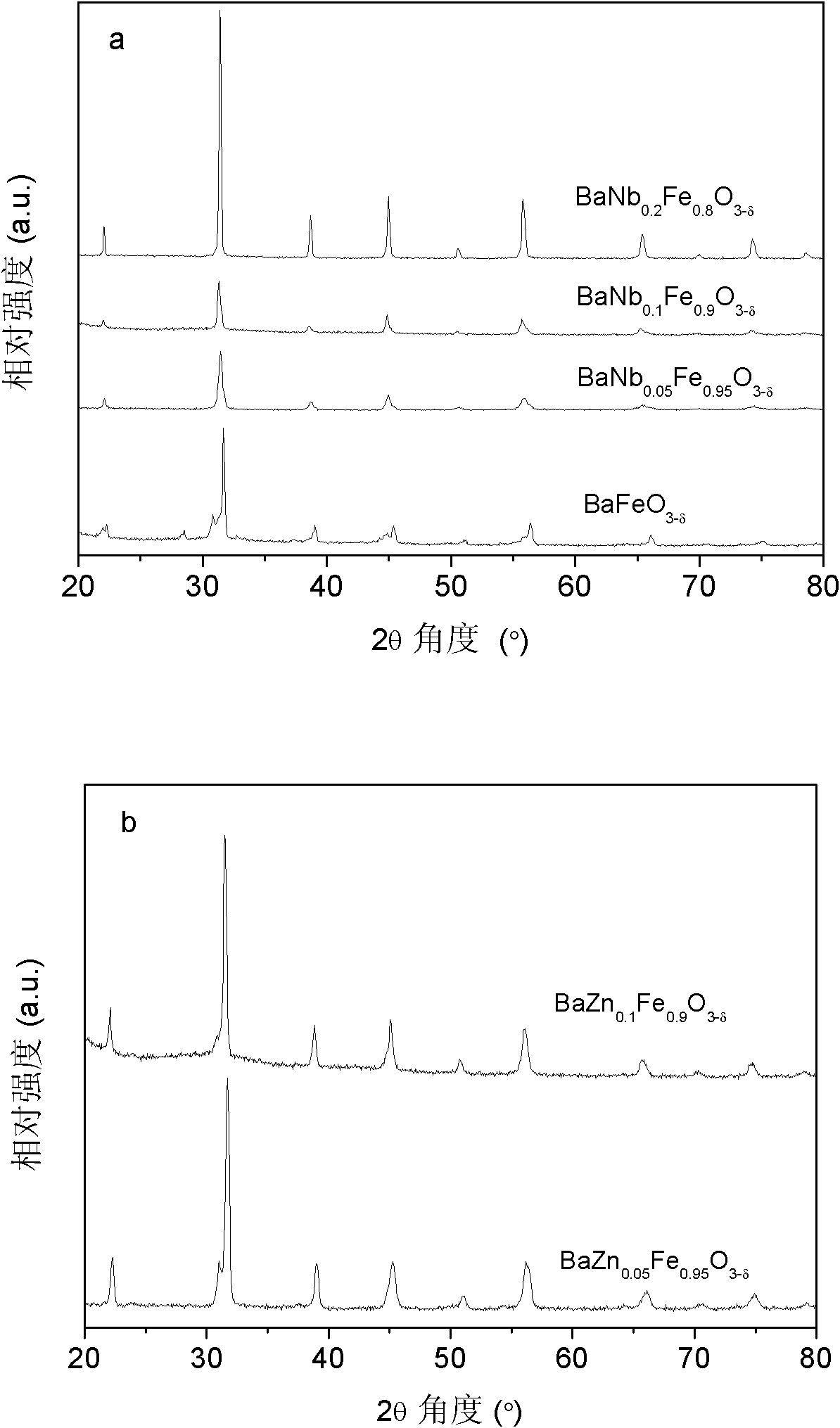
[0022] BaNb 0.05 Fe 0.95 o 3-δ Synthesized by high-temperature solid-phase method. According to the target product ratio, the stoichiometric ratio of BaCO 3 (analytical pure), Nb 2 o 3 (analytical pure), Fe 2 o 3 (analytical pure) mixed, using alcohol or acetone as the medium, ball milled in a high-energy ball mill (FRITSCH, Pulverisette 6) for 1 hour until the mixture is uniform, and the mixture is further evaporated and solidified at 250 ° C. After drying is complete, the precursor body, and finally bake the precursor at 1250°C for 10h to obtain the desired BaNb 0.05 Fe 0.95 o 3-δ Cathode material, wherein -0.5figure 1 The XRD powder diffraction method shown in (a) shows that BaNb 0.05 Fe 0.95 o 3-δ A phase-pure perovskite structure is formed.
Embodiment 2
[0023] Example 2: BaNb 0.1 Fe 0.9 o 3-δ Preparation of cathode material (solid phase method)
[0024] BaNb 0.1 Fe 0.9 o3-δ Synthesized by high-temperature solid-phase method. According to the target product ratio, the stoichiometric ratio of BaCO 3 (analytical pure), Nb 2 o 3 (analytical pure), Fe 2 o 3 (analytical pure) mixed, using alcohol or acetone as the medium, ball milled in a high-energy ball mill (FRITSCH, Pulverisette 6) for 2 hours until the mixture is uniform, and the mixture is further evaporated and solidified at 240 ° C. After drying is complete, the precursor body, and finally bake the precursor at 1300°C for 5h to obtain the desired BaNb 0.1 Fe 0.9 o 3-δ Cathode material, wherein -0.5figure 1 The XRD powder diffraction method shown in (a) shows that BaNb 0.1 Fe 0.9 o 3-δ A phase-pure perovskite structure is formed.
Embodiment 3
[0025] Example 3: BaNb 0.2 Fe 0.8 o 3-δ Preparation of cathode material (solid phase method)
[0026] BaNb 0.2 Fe 0.8 o 3-δ Synthesized by high-temperature solid-phase method. According to the target product ratio, the stoichiometric ratio of BaCO 3 (analytical pure), Nb 2 o 3 (analytical pure), Fe 2 o 3 (analytical pure) mixed, using alcohol or acetone as the medium, ball milled in a high-energy ball mill (FRITSCH, Pulverisette 6) for 3 hours until the mixture is uniform, and the mixture is further evaporated and solidified at 200 ° C. After drying is complete, the precursor body, and finally bake the precursor at 1350°C for 20h to obtain the desired BaNb 0.2 Fe 0.8 o 3-δ Cathode material, wherein -0.5figure 1 The XRD powder diffraction method shown in (a) shows that BaNb 0.2 Fe 0.8 o 3-δ A phase-pure perovskite structure is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com