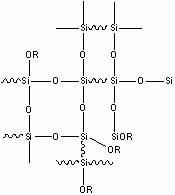
Organosilicon type marine antifouling paint binder and preparation method and application thereof
A marine antifouling and silicone technology, applied in antifouling/underwater coatings, polyurea/polyurethane coatings, coatings, etc., to avoid the use of heavy metal ions and to achieve the effect of good environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Add 30g of dimethyldimethoxysilane, 68.47g of chloropropylmethyldimethoxysilane, 0.15g of solid acid clay, 5g of water into a 250ml three-necked flask, hydrolyze and polymerize for 3 hours at 80°C, and filter Remove acid clay, distill off water and methanol to obtain chloropropyl polysiloxane;
[0028] 2) Add 10g of chloropropyl polysiloxane synthesized in step (1) into a 250ml three-necked flask equipped with a reflux condenser, a stirring device, and a thermometer, add 50ml of n-butanol, 9.5g of tertiary dodecylamine and 1.06g of N methyldiethanolamine, under the protection of nitrogen, reflux reaction at 120°C for 10h, after the reaction was completed, the solvent was distilled off under reduced pressure to obtain polysiloxane containing quaternary ammonium salt and hydroxyl group. The content of quaternary ammonium salt is determined by cationic titration, the indicator is methylene blue, and the quaternization yield is 99%;
[0029] 3) Add the polysiloxane cont...
Embodiment 2
[0031] 1) Add 30g of dimethyldimethoxysilane, 45.50g of chloropropylmethyldimethoxysilane, 0.15g of solid acid clay, 5g of water into a 250ml three-neck flask, conduct a hydrolysis polymerization reaction at 80°C for 3 hours, and remove by filtration Acid clay, water and methanol are distilled off to obtain chloropropyl polysiloxane;
[0032] 2) Add 10g of chloropropyl polysiloxane synthesized in step (1) into a 250ml four-necked flask equipped with a reflux condenser, a stirring device, and a thermometer, add 50ml of n-butanol, and 8.10g of tertiary dodecylamine And 1.13g N methyldiethanolamine, under the protection of nitrogen, reflux reaction at 120°C for 10h, after the reaction was completed, the solvent was distilled off under reduced pressure to obtain polysiloxane containing quaternary ammonium salt and hydroxyl group. The content of quaternary ammonium salt is determined by cationic titration, the indicator is methylene blue, and the quaternization yield is 99%;
[00...
Embodiment 3
[0035] 1) Add 30g of dimethyldimethoxysilane, 30.43g of chloropropylmethyldimethoxysilane, 0.15g of acid clay, 5g of water into a 250ml three-neck flask, hydrolyze and polymerize for 3 hours at 80°C, and remove the acid by filtration White clay, water and methanol are distilled off to obtain chloropropyl polysiloxane;
[0036] 2) Add 10g of chloropropyl polysiloxane synthesized in step (1) into a 250ml four-neck flask equipped with a reflux condenser, a stirring device and a thermometer, add 50ml of n-butanol, and 6.45g of tertiary dodecylamine and 1.20 g of N methyldiethanolamine, under the protection of nitrogen, reflux at 120° C. for 10 h. After the reaction, the solvent was distilled off under reduced pressure to obtain a polysiloxane containing a quaternary ammonium salt and a hydroxyl group. The content of quaternary ammonium salt is determined by cationic titration, the indicator is methylene blue, and the quaternization yield is 99%;
[0037] 3) Add the polysiloxane c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com