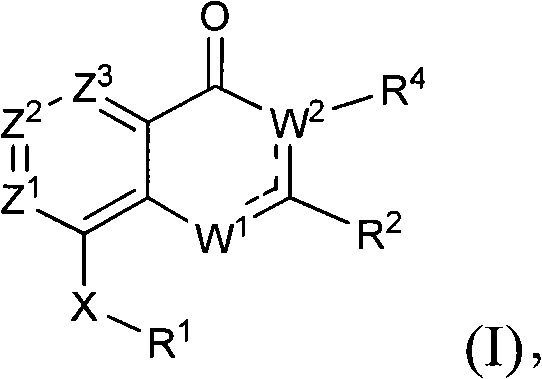
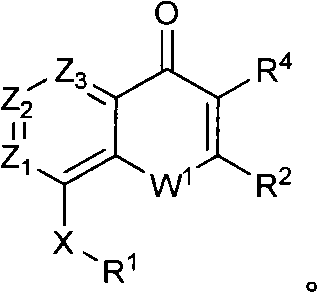
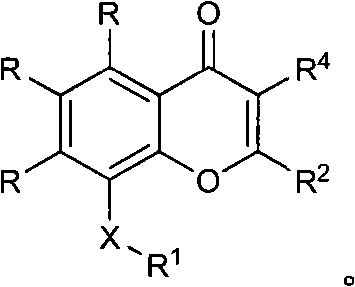
Quinazolinone, quinolone and related analogs as sirtuin modulators
A compound, alkyl technology, applied in the field of quinazolinones, quinolones and related analogs as sirtuin regulators, which can solve the problems of reducing and extending the lifespan of wild-type cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0334] Example 1. N-(3-methyl-4-oxo-2-(3-(trifluoromethyl)phenyl)-3,4-dihydroquinazolin-8-yl) Synthesis of pyridine-2-carboxamide (compound 239):
[0335] Step 1. 8-Nitro-2-(3-(trifluoromethyl)phenyl)-4H-benzo[d][1,3] Preparation of oxazin-4-one (3):
[0336]
[0337] 3-(Trifluoromethyl)benzoyl chloride 2 (4.5 mL, 30.2 mmol) was added to a suspension of 2-amino-3-nitrobenzoic acid 1 (5.0 g, 27.5 mmol) in pyridine (65 mL). The reaction mixture was stirred at room temperature for 12 h, then poured into ice-cooled H 2 O (300mL). The resulting precipitate was collected by filtration and washed with H 2 O washed and dried in vacuo to afford 3 (5 g, 51% yield) as a yellow solid which was used without further purification.
[0338] Step 2. Preparation of 3-methyl-8-nitro-2-(3-(trifluoromethyl)phenyl)quinazolin-4(3H)-one (4):
[0339]
[0340]Methylamine (6.3 mL, 12.5 mmol) in THF was added to 8-nitro-2-(3-(trifluoromethyl)phenyl)-4H-benzo[d][1,3] In a suspension of a...
Embodiment 2
[0347] Example 2. N-(3-methyl-4-oxo-2-phenyl-3,4-dihydroquinazolin-8-yl)pyridine-3-sulfonamide Synthesis of (Compound 361):
[0348]
[0349] Pyridine-3-sulfonyl chloride hydrochloride 7 (280 mg, 1.3 mmol) was added to 8-amino-3-methyl-2-(3-(trifluoromethyl)phenyl)quinazoline-4(3H)- Ketone 5 (100 mg, 0.313 mmol) in pyridine (5 mL). The reaction mixture was heated at 80°C for 12 hours. Pyridine was removed in vacuo. The residue was dissolved in CH 2 Cl 2 , with saturated NaHCO 3 Washed with aqueous solution, dried (MgSO 4 ) and concentrate. The crude residue was purified by MPLC with CH 2 Cl 2 / MeOH (0-10%) elution followed by CH 3 CN was recrystallized to give compound 361 (77 mg, 53% yield). MS(ESI) calculated value C 21 h 15 f 3 N 4 o 3 S: 460.08; found value: 461 [M+H].
Embodiment 3
[0350] Example 3. N-(4-oxo-2-(3-(trifluoromethyl)phenyl)-3,4-dihydroquinazolin-8-yl)pyridine-2- Synthesis of formamide (compound 231):
[0351] Step 1. Preparation of 8-nitro-2-(3-(trifluoromethyl)phenyl)quinazolin-4(3H)-one (8):
[0352]
[0353] 8-nitro-2-(3-(trifluoromethyl)phenyl)-4H-benzo[d][1,3] Azin-4-one 3 (150 mg, 0.45 mmol) was added to a solution of ammonia (5.0 mL, 10 mmol) in IPA. The reaction mixture was heated at gentle reflux for 12 hours and then cooled to room temperature. Pour the reaction mixture into H 2 O, the resulting precipitate was collected by filtration and washed with H 2 O washed and dried in vacuo. The crude residue was recrystallized from EtOAc to afford 8 (91 mg, 62% yield) as a yellow solid.
[0354] Step 2. Preparation of 8-amino-2-(3-(trifluoromethyl)phenyl)quinazolin-4(3H)-one (9):
[0355]
[0356] Compound 9 was prepared in 99% yield by a method analogous to the preparation of 8-amino-3-methyl-2-(3-(trifluoromethyl)phenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com