Application of Coryebacterium parvum or its composition in preparing medicaments for treating hepatitis B
A technology for Corynebacterium brevis and hepatitis B, which is applied in the preparation of medicaments for the treatment of hepatitis B, and the application field of the preparation of medicaments for the treatment of hepatitis B, can solve the problem of increasing the proportion of virus resistance variation, High mutation rate, aggravation of disease and other problems, to achieve the effect of improved absorption and diffusivity, uniform particle size, and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
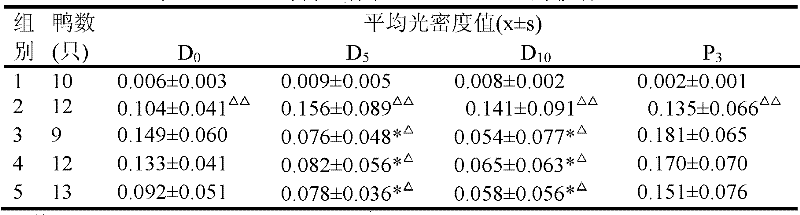
[0063] Experimental Example 1 Protective effect of NCPP on Beijing shelduck duck hepatitis B infection:
[0064] 1. Materials and methods
[0065] 1. Materials
[0066] 1.1 Animals: 150 1-day-old Peking ducks, male or female, purchased from Beijing Qianjin Duck Factory.
[0067] 1.2 Duck hepatitis B virus positive serum: collected from Shanghai shelduck, donated by Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences, stored at -20°C.
[0068] 1.3 Plasmid: pUC18-DHBV-DNA (containing the whole genome DNA of DHBV), donated by the Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences, stored at -20°C.
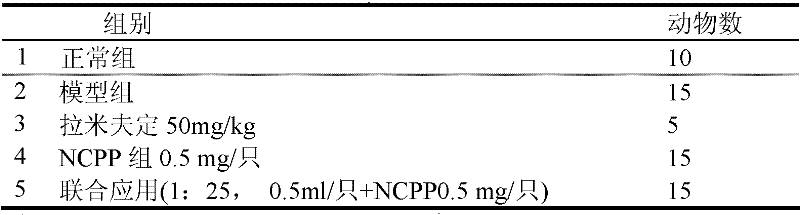
[0069] 1.4 Test product:
[0070] Test product 1: acellular brevus preparation (NCPP), properties: white homogeneous opaque liquid, provided by China National Institute for the Control of Pharmaceutical and Biological Products.
[0071] Test product 2: Hepatitis B immunoglobulin, provided by China Institute for the Control of ...
Embodiment 2
[0128] The protective effect of embodiment 2CPP on Beijing shelduck duck hepatitis B infection
[0129] 1. Materials and methods
[0130] 1. Materials
[0131] 1.1 Animals: 150 1-day-old Peking ducks, male or female, purchased from Beijing Qianjin Duck Factory.
[0132] 1.2 Duck hepatitis B virus positive serum: collected from Shanghai shelduck, donated by Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences, stored at -20°C.
[0133] 1.3 Plasmid: pUC18-DHBV-DNA (containing the whole genome DNA of DHBV), donated by the Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences, stored at -20°C.
[0134] 1.4 Test product:
[0135] Test product 1: Corynebacterium brevis preparation (CPP), properties: white homogeneous opaque liquid, provided by China National Institute for the Control of Pharmaceutical and Biological Products.
[0136] Test product 2: Hepatitis B immunoglobulin, provided by China Institute for the Control of Phar...
Embodiment 3
[0160] Embodiment 3 clinical trials
[0161] 1. Treatment objects and methods
[0162] 1.1 Inclusion criteria: age 12-65 years old, body weight ≥ 33kg, positive serum HBsAg and hepatitis B virus (HBV) DNA > 6 months before screening, positive HBsAg and HBV-DNA during screening (dot blot method) , Serum alanine aminotransferase (alanine aminotransferase, ALT) within 3 months before screening is below 10 times the upper limit of normal (ULN).
[0163] 1.2 Exclusion criteria: anti-HCV, anti-HDV and anti-HIV positive; decompensated chronic liver disease; suspected hepatocellular carcinoma, autoimmune liver disease, genetic liver disease; bone marrow suppression; abnormal renal function; severe organic disease disease, mental illness; alcoholism, drug abuse; used antiviral drugs (including lamivudine), immunomodulators or inhibitors, cytotoxic drugs or steroid hormones 6 months before screening; Those who have not taken effective contraceptive measures and those with a history of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com