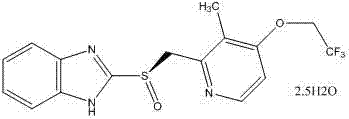
Lansoprazole compound
A technology of lansoprazole hydrate and dexlansoprazole, which is applied in the field of dexlansoprazole compound and its preparation, can solve the problem of high total amount of impurities, large number of impurities of dexlansoprazole, and low optical purity And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]In the 1000ml reaction flask equipped with stirring, thermometer and condenser, add 80 grams of dexlansoprazole and 400ml of isopropanol-acetic acid-water=5-6:1-2:3-4 in the mixed solution, Stirring was started, heated to 70°C-72°C, filtered while hot, naturally cooled to room temperature, and kept warm for 5 hours, crystals were precipitated, filtered, and dried indoors to obtain 72.7 grams of white crystals. Determined by Karl Fischer method, it contains 10.88% (weight percent) of water. Purity 99.95% (HPLC normalization method), optical purity 99.99% ee.
[0052] Elemental Analysis Results:
[0053] Measured value (calculated value), C:46.28(46.37),H:4.60(4.62),N:10.20(10.14),
[0054] S: 7.79 (7.74), F: 13.81 (13.75).
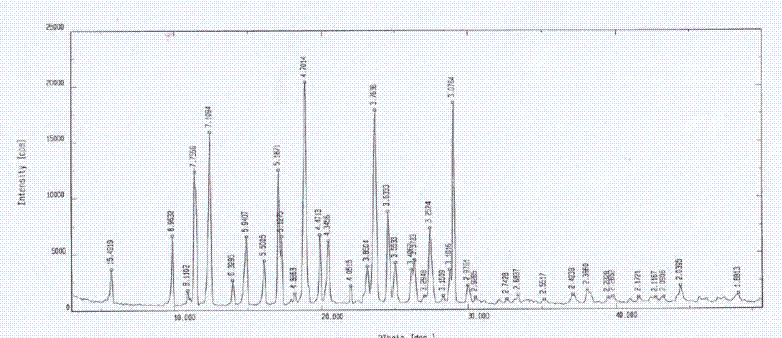
[0055] The X-ray diffraction pattern of the crystal is shown in figure 1 . Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° ;
Embodiment 2
[0058] Capsules containing two hemihydrates of dexlansoprazole
[0059] Prescription: 60 grams of dexlansoprazole two hemihydrates, 5 ml of propylene glycol, and 150 grams of starch, made into 1000 capsules.
[0060] Process: Moisten two hemihydrates of dexlansoprazole and starch with 15% propylene glycol aqueous solution, mix well, sieve and granulate, dry at 60°C, granulate, and fill capsules.
Embodiment 3
[0062] Tablets containing two hemihydrates of dexlansoprazole
[0063] Prescription: 30 grams of dexlansoprazole two hemihydrates, 200 grams of lactose, 30 grams of PEG-4000, 11 grams of magnesium stearate, 27 grams of povidone K30, 50 grams of croscarmellose sodium, Appropriate amount of distilled water, made into 1000 tablets.
[0064] Process: Grind PEG-4000 and two hemihydrates of dexlansoprazole together, pass through 80-mesh sieve, mix with other materials, make soft material with distilled water, make granules with 16-mesh sieve, put in a drying box at 40-45 Dry at ℃, granulate with a 16-mesh sieve, add magnesium stearate to the dry granules, mix evenly, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com