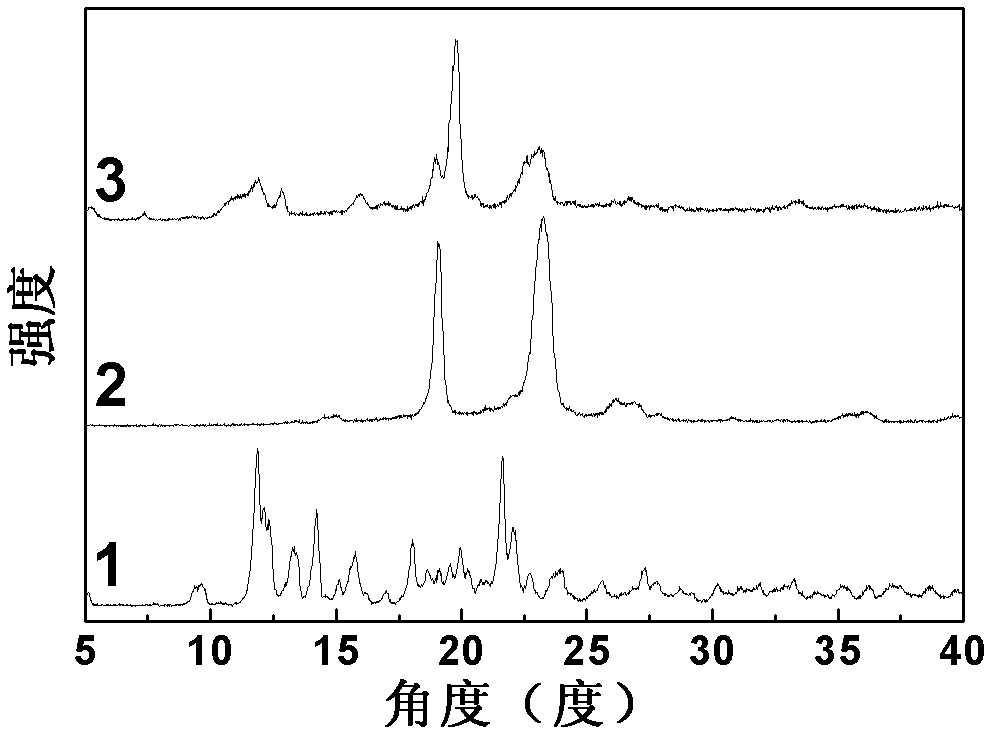
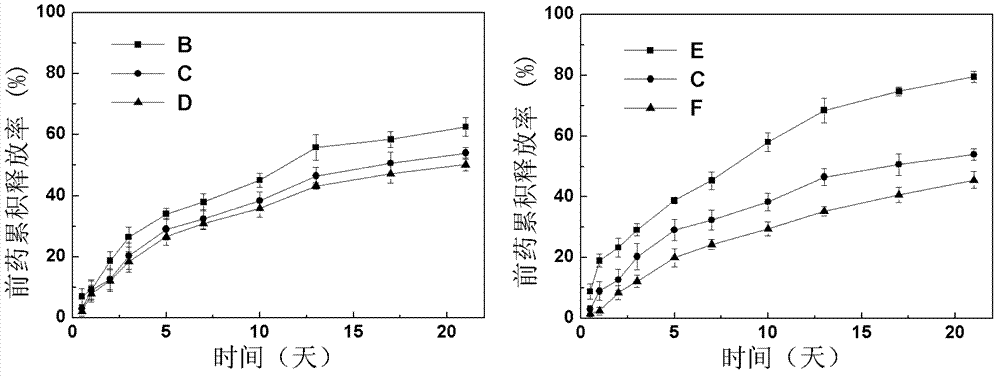
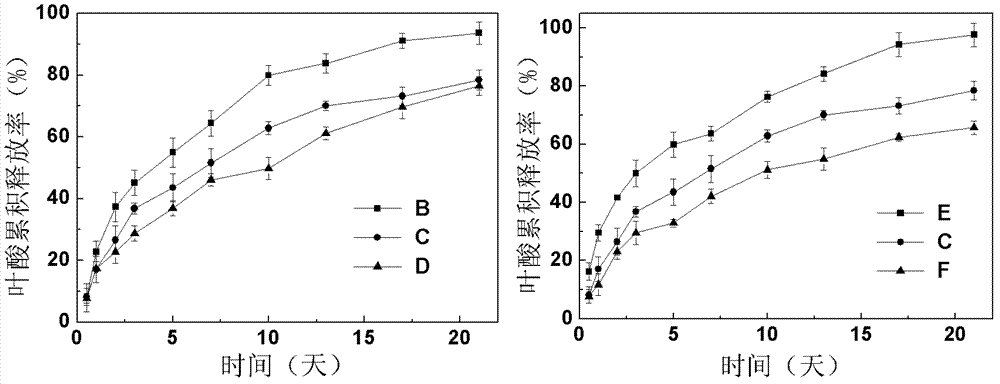
Super-molecular hydrogel double-medicament carrier and preparation method as well as application thereof
A supramolecular hydrogel, hydrophobic drug technology, applied in the field of biomedical engineering materials, achieves the effect of avoiding residue, shortening gelation time, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dry polyethylene glycol monomethyl ether (MPEG, molecular weight 2000) and indomethacin (IND) were dissolved in anhydrous dimethyl sulfoxide under stirring conditions of 300 rpm to obtain a solution ( 100 ml of dimethyl sulfoxide contains 10 g of polyethylene glycol monomethyl ether); add 1,3-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) to the solution, 5 Stirring reaction at ℃ for 48 hours; the molar ratio of polyethylene glycol monomethyl ether, hydrophobic drug, 1,3-dicyclohexylcarbodiimide and 4-dimethylaminopyridine is 1:1.1:1.2:1.1 After the reaction is completed, the product is filtered, and the filtrate is precipitated with ether; the resulting precipitate is redissolved in water, filtered and dialyzed for 2 days using a dialysis bag with a molecular weight cut-off of 3000, and freeze-dried to obtain prodrug A (MPEG-IND).
Embodiment 2
[0037]Dry polyethylene glycol monomethyl ether (MPEG, molecular weight 10000) and indomethacin (IND) were dissolved in anhydrous dimethyl sulfoxide under stirring conditions of 900 rpm to obtain a solution ( 20 g of polyethylene glycol monomethyl ether per 100 ml of dimethyl sulfoxide); 1,3-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) were added to the solution, 35 Stirring reaction at ℃ for 24 hours; the molar ratio of polyethylene glycol monomethyl ether, hydrophobic drug, 1,3-dicyclohexylcarbodiimide and 4-dimethylaminopyridine is 1:1.1:1.2:1.1 After the reaction is completed, the product is filtered, and the filtrate is precipitated with ether; the resulting precipitate is redissolved in water, filtered and dialyzed for 3 days using a dialysis bag with a molecular weight cut-off of 500, and freeze-dried to obtain the prodrug (MPEG-IND).
Embodiment 3
[0039] Under the condition of room temperature 25 DEG C, the obtained MPEG-IND of embodiment 1 is dissolved in the phosphate buffer solution that pH is 6 and is made into the prodrug solution that the mass percent concentration is 8%, and the folic acid solution that the mass percent concentration is 1.0% ( Folic acid is dissolved in the sodium bicarbonate solution of 0.1mol / L and obtains) mixes in equal volumes, then adds the α-cyclodextrin solution (α-cyclodextrin Dissolved in a phosphate buffer solution with a pH of 6), stirred at a speed of 60 rpm for 60 seconds, mixed evenly, and stood at 5°C for 12 hours to obtain a supramolecular hydrogel loaded with both hydrophilic and hydrophobic drugs Gum Dual Drug Carrier B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com