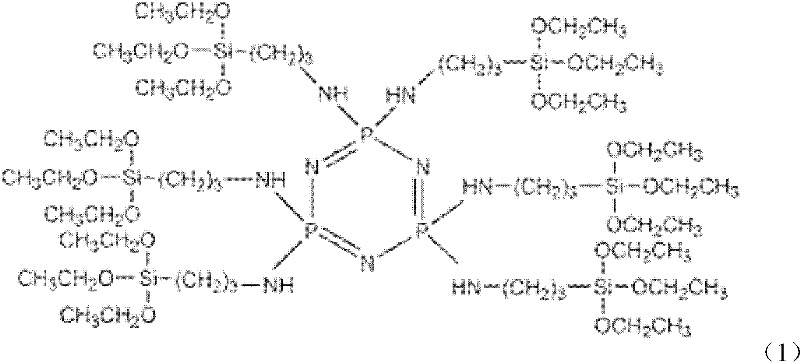
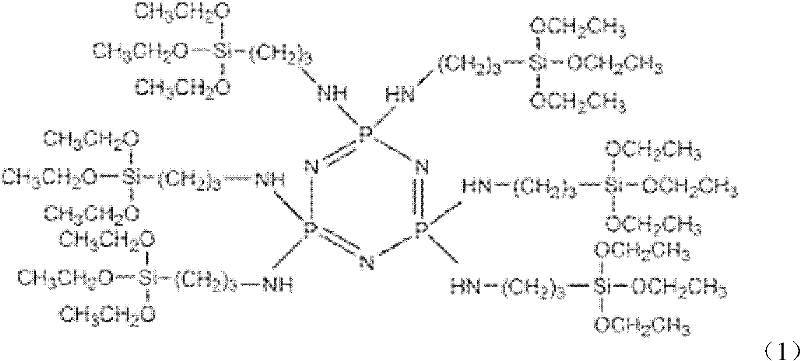
Preparation method and application of cyclotriphosphazene containing silicon functional groups
A technology of cyclotriphosphazene and hexachlorocyclotriphosphazene is applied in the field of flame retardant, which can solve the problems of low yield and the like, and achieve the effects of high yield, high elongation at break and high oxygen index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Add 14.61g of aminopropyltriethoxysilane and 7.28g of triethylamine into 20ml of tetrahydrofuran solvent, stir and mix evenly, pass in argon, and slowly heat to 60°C;
[0024] 2) 3.48g of hexachlorocyclotriphosphazene was dissolved in 10ml of tetrahydrofuran solvent, magnetically stirred to make it fully dissolved, and then added dropwise to the solution in step 1), a white precipitate was gradually formed, and the dropping time was 30min, at 67 The reaction was carried out at ℃ for 10 hours; after the reaction, the obtained product was suction filtered to remove the white triethylamine hydrochloride, and then the filtrate was rotated in vacuum to remove the tetrahydrofuran solvent and excess triethylamine to obtain the silicon-containing functional group cyclotriethylamine hydrochloride. Phosphazene is a light yellow-green liquid with a yield of 90.4%.
[0025] Spectral data for phosphazene compounds containing organosilicon groups are:
[0026] FT-IR (cm -1 ): 32...
Embodiment 2
[0030] 1) Add 15.49g of aminopropyltriethoxysilane and 7.28g of triethylamine into 20ml of tetrahydrofuran solvent, stir and mix evenly, pass in argon, and slowly heat to 60°C;
[0031] 2) 3.47g of hexachlorocyclotriphosphazene was dissolved in 10ml of tetrahydrofuran solvent, magnetically stirred to make it fully dissolved, and then added dropwise to the solution in step 1), a white precipitate was gradually formed, and the time for adding was 30min. The reaction was carried out at ℃ for 10 hours; after the reaction, the obtained product was suction filtered to remove the white triethylamine hydrochloride, and then the filtrate was rotated in vacuum to remove the tetrahydrofuran solvent and excess triethylamine to obtain the silicon-containing functional group cyclotriethylamine hydrochloride. Phosphazene is a light yellow-green liquid with a yield of 93.5%.
[0032] Compound the above-mentioned 6g of silicon-containing functional group cyclotriphosphazene, 24g of ammonium po...
Embodiment 3
[0035] 1) Add 14.60g of aminopropyltriethoxysilane and 8.11g of triethylamine into 20ml of tetrahydrofuran solvent, stir and mix evenly, pass in argon, and slowly heat to 60°C;
[0036] 2) 3.48g of hexachlorocyclotriphosphazene was dissolved in 10ml of tetrahydrofuran solvent, magnetically stirred to make it fully dissolved, and then added dropwise to the solution in step 1), a white precipitate was gradually formed, and the dropping time was 30min, at 67 The reaction was carried out at ℃ for 10 hours; after the reaction, the obtained product was suction filtered to remove the white triethylamine hydrochloride, and then the filtrate was rotated in vacuum to remove the tetrahydrofuran solvent and excess triethylamine to obtain the silicon-containing functional group cyclotriethylamine hydrochloride. Phosphazene is a light yellow-green liquid with a yield of 91.4%.
[0037] Compound the 4g of silicon-containing functional cyclotriphosphazene, 26g of ammonium polyphosphate and 70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com