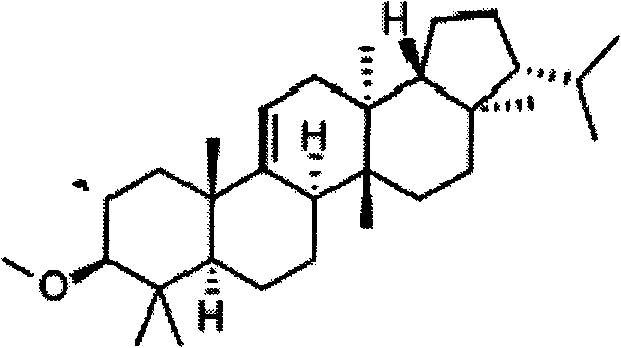
Method for preparing high-purity arundoin
A high-purity technology of arunecillin, which is applied in the field of separation of effective components of natural plants, can solve the problems of extraction process reports on the pharmacological effects of arunecilin, and the lack of arunecilin, and achieve large preparation, short separation time, and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Grind 1.5kg of the rhizome of Imperatae Rhizoma, soak in 80% ethanol at 60°C for 0.5 hour, reflux extraction for 2 hours, extract twice, combine the extracts, concentrate the extracts under reduced pressure to obtain an ethanol extract. The ethanol extract was extracted with chloroform, and the chloroform layer was concentrated under reduced pressure to obtain 8.7 g of extract. The chloroform extract was subjected to silica gel column chromatography, chloroform-acetone (1:1.5) isocratic gradient elution, the eluate flowing out in 2-4 column bed volumes was collected, the solvent was recovered, and crystallized with chloroform-acetone to obtain Coarse crystal 5.3g. 200 mg of crystals were separated by high-speed countercurrent chromatography, and the solvent system n-hexane-ethyl acetate-methanol-water was selected. First, the above-mentioned solvent system was configured in a separatory funnel at a volume ratio of 8:2:1.5:0.5, and shaken well After standing for stratif...
Embodiment 2
[0014] Grind 1.5 kg of Rhizoma Imperatae medicinal material, soak in 95% ethanol at 60°C for 0.5 hour, reflux extraction for 1.5 hour, extract twice, combine extracts, concentrate under reduced pressure to obtain ethanol extract. The ethanol extract was extracted with chloroform, and the chloroform layer was concentrated under reduced pressure to obtain 8.5 g of extract. The chloroform extract was subjected to silica gel column chromatography, chloroform-acetone (1:1.5) isocratic gradient elution, the eluate flowing out in 2-4 column bed volumes was collected, the solvent was recovered, and crystallized with chloroform-acetone to obtain Coarse crystal 5.0g. 200 mg of crystals were separated by high-speed countercurrent chromatography, and the solvent system n-hexane-ethyl acetate-methanol-water was selected. First, the above-mentioned solvent system was arranged in a separatory funnel at a volume ratio of 10:5:2.5:1, and shaken evenly After standing for stratification, separa...
Embodiment 3
[0016] Grind 1.5 kg of Rhizoma Imperatae medicinal material, soak in 85% ethanol at 60°C for 0.5 hour, reflux extraction for 1.5 hour, extract twice, combine extracts, concentrate extracts under reduced pressure to obtain ethanol extract. The ethanol extract was extracted with chloroform, and the chloroform layer was concentrated under reduced pressure to obtain 8.4 g of extract. The chloroform extract was subjected to silica gel column chromatography, chloroform-acetone (1:1.5) isocratic gradient elution, the eluate flowing out in 2-4 column bed volumes was collected, the solvent was recovered, and crystallized with chloroform-acetone to obtain Coarse crystal 5.1g. 250 mg of crystals were separated by high-speed countercurrent chromatography, and the solvent system n-hexane-ethyl acetate-methanol-water was selected. First, the above-mentioned solvent system was arranged in a separatory funnel at a volume ratio of 13:3.5:4:2, and shaken well After standing for stratification,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com