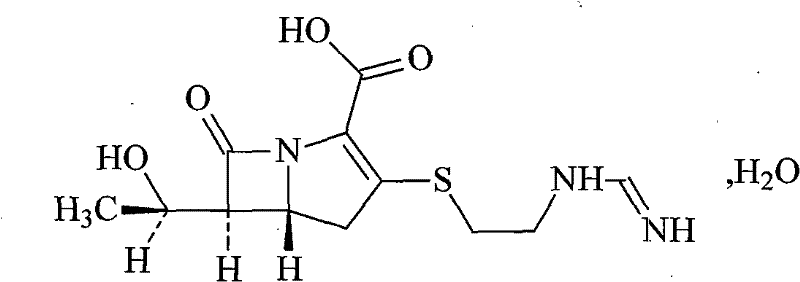
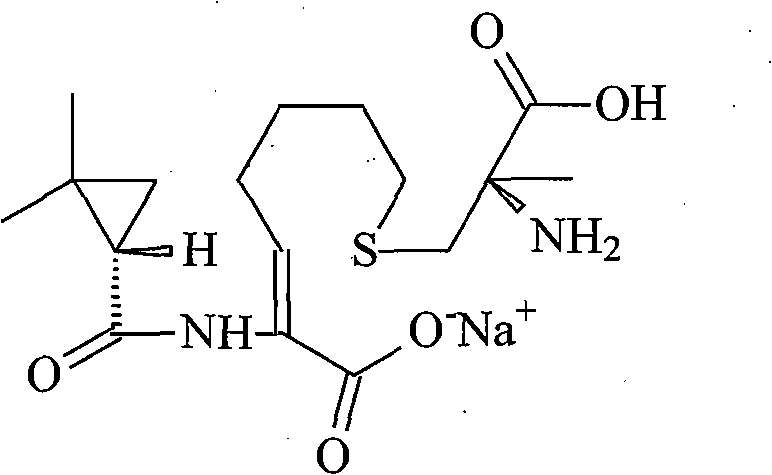
A kind of imipenem cilastatin sodium pharmaceutical composition liposome injection
A technology of imipenem cilastatin sodium and cilastatin sodium, which is applied in the field of medicine, can solve problems such as unsolved oxidative decomposition of components, accurate proportioning of content, and impact on the quality of final products, so as to avoid organic Solvent toxicity, small particle size, and the effect of improving encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Imipenem cilastatin sodium pharmaceutical composition liposome injection
[0039] Prescription (1000 bottles):
[0040]
[0041] Preparation Process:
[0042] (1) 150g sphingomyelin, 50g cholesterol, 50g Pluronic F-68 are dissolved in the phosphate buffered saline solution of pH6.5 of 600ml, make blank heterogeneous liposome;
[0043] (2) Dissolve 100g of imipenem and 100g of cilastatin sodium in 400ml of pH 6.5 phosphate buffer solution, stir to dissolve completely;
[0044] (3) After the blank liposome prepared in step (1) and the imipenem cilastatin sodium buffered phosphate solution in step (2) are sterilized by circulating steam, the sterilized solution is treated twice by ultrasonic method , 10 minutes each time;
[0045](4) Under sterile conditions, add imipenem cilastatin sodium buffer solution to the blank liposomes in a molten state at 60°C, stir, then add 150g trehalose and 50g PVP, continue stirring to dissolve it, and obtain Imipenem cilast...
Embodiment 2
[0047] Example 2 Imipenem cilastatin sodium pharmaceutical composition liposome injection
[0048] Prescription (1000 bottles):
[0049]
[0050] Preparation Process:
[0051] (1) 175g sphingomyelin, 100g cholesterol, 75g Pluronic F-68 are dissolved in the phosphate buffered saline solution of pH6.5 of 800ml, make the multiphase liposome of blank;
[0052] (2) Dissolve 50g of imipenem and 50g of cilastatin sodium in 200ml of pH 6.5 phosphate buffer solution, and stir to make it completely dissolved;
[0053] (3) After the blank liposome prepared in step (1) and the imipenem cilastatin sodium buffered phosphate solution in step (2) are sterilized by circulating steam, the sterilized solution is treated twice by ultrasonic method , 10 minutes each time;
[0054] (4) Under sterile conditions, add imipenem cilastatin sodium buffer solution to the blank liposomes in a molten state at 60°C, stir, then add 200g trehalose and 100g PVP, continue stirring to dissolve it, and obtai...
Embodiment 3
[0056] Example 3 Imipenem cilastatin sodium pharmaceutical composition liposome injection
[0057] Prescription (1000 bottles):
[0058]
[0059] Preparation Process:
[0060] (1) 62.5g sphingomyelin, 25g cholesterol, 50g Pluronic F-68 are dissolved in the phosphate buffered saline solution of pH6.5 of 400ml, make the multiphase liposome of blank;
[0061] (2) Dissolve 25g of imipenem and 25g of cilastatin sodium in 100ml of pH6.5 phosphate buffer solution, stir to make it completely dissolve;
[0062] (3) After the blank liposome prepared in step (1) and the imipenem cilastatin sodium buffered phosphate solution in step (2) are sterilized by circulating steam, the sterilized solution is treated twice by ultrasonic method , 10 minutes each time;
[0063] (4) Under sterile conditions, add imipenem cilastatin sodium buffer solution to the blank liposomes in the molten state at 60°C, stir, then add 75g trehalose and 25g PVP, continue stirring to dissolve it, and obtain Imi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com