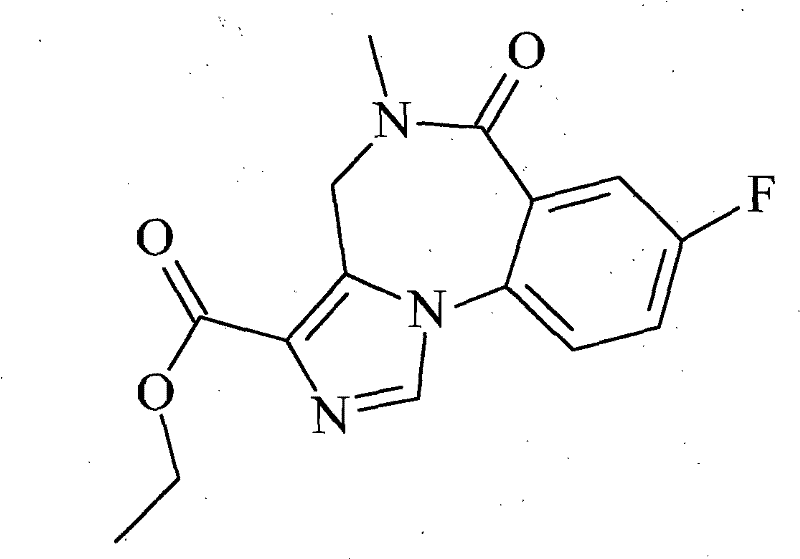
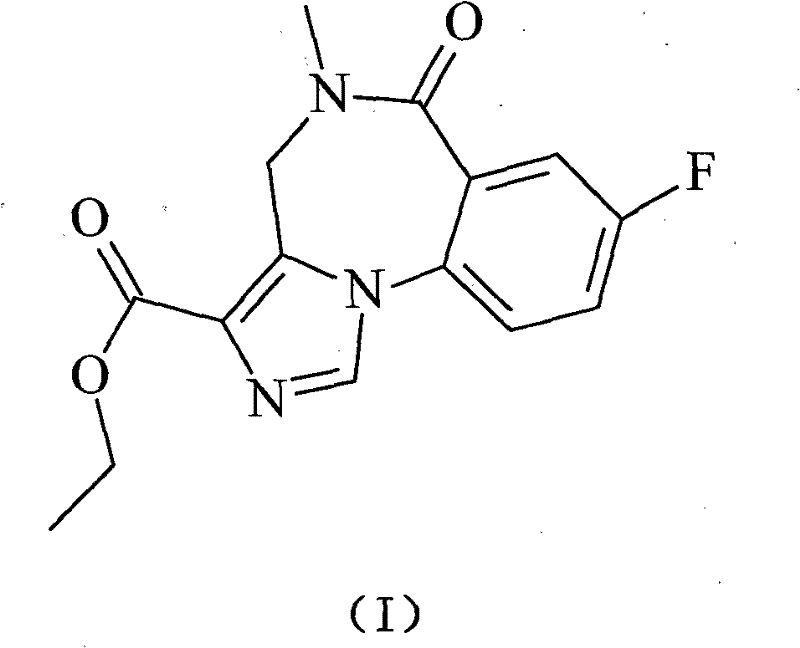
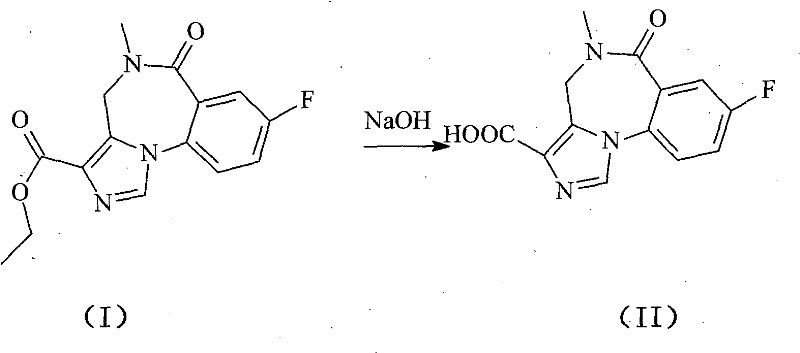
A kind of flumazenil compound and its preparation method
A flumazenil and compound technology, applied in the field of flumazenil compounds and preparation methods thereof, can solve problems such as difficulty in obtaining high-purity and high-yield flumazenil compounds, reduce toxic and side effects, improve product quality, improve reaction simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The purification of embodiment 1 flumazenil
[0036] (1) Disperse 100g of flumazenil crude product with a purity of 98.24% in 500ml of water, add 200ml of sodium hydroxide solution with a mass percentage of about 6% as a catalyst, heat to 45°C, stir and react for 2 hours, and adjust the pH to 5.5, extracted with 500ml of diethyl ether, dried with a solid desiccant, filtered, added 0.5g of activated carbon, kept at 40°C for 30 minutes, stirred and adsorbed, decarburized by filtration, distilled off under reduced pressure to remove diethyl ether to obtain 83.15g of intermediate (II);
[0037] (2) Add 16.8g of ethanol to the intermediate (II), then add 2.6g of copper chloride (CuCl 2 2H 2 O) as catalyzer, 26ml hexanaphthene is used as water-carrying agent, under stirring heating reflux, stop reaction when no water comes out in water separator, filter out insoluble matter, filtrate is dried with activated alumina again, removes diethyl ether by distillation under reduced p...
Embodiment 2
[0038] The purification of embodiment 2 flumazenil
[0039] (1) Disperse 100g of flumazenil crude product with a purity of 98.24% in 500ml of water, add 200ml of sodium hydroxide solution with a mass percentage of about 6% as a catalyst, heat to 55°C, stir and react for 2 hours, and adjust the pH to 6.0, extracted with 500ml of diethyl ether, dried with a solid desiccant, filtered, added 2.5g of activated carbon, kept at 45°C for 30 minutes, stirred and adsorbed, decarburized by filtration, distilled off under reduced pressure to obtain 83.24g of intermediate (II);
[0040](2) Add 18.8g of ethanol to the intermediate (II), then add 3.9g of copper chloride (CuCl 2 2H 2 O) as catalyzer, 29ml hexanaphthene is used as water-carrying agent, under stirring heating reflux, stop reaction when no water comes out in water trap, filter out insoluble matter, filtrate is dried with activated alumina again, diethyl ether is removed by distillation under reduced pressure, After vacuum dryi...
Embodiment 3
[0041] The purification of embodiment 3 flumazenil
[0042] (1) Disperse 100g of flumazenil crude product with a purity of 98.24% in 500ml of water, add 200ml of sodium hydroxide solution with a mass percentage of about 6% as a catalyst, heat to 60°C, stir and react for 2.5 hours, and adjust the pH to 6.5, extracted with 500ml of diethyl ether, dried with solid desiccant, filtered, added 1g of activated carbon, kept at 50°C for 30 minutes, stirred and adsorbed, decarburized by filtration, and the organic solvent was distilled off under reduced pressure to obtain 81.68g of intermediate (II);
[0043] (2) Add 20.5g ethanol to intermediate (II), then add 5.1g copper chloride (CuCl 2 2H 2 O) as catalyzer, 32ml hexanaphthene is as water-carrying agent, reflux under heating under agitation, stop reaction when anhydrous goes out in water separator, filter out insoluble matter, filtrate is dried with activated alumina again, diethyl ether is removed by distillation under reduced pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com