A kind of minodronic acid crystal form ii and preparation method thereof
A technology of minodronic acid and crystal form, applied in the field of drug synthesis, can solve the problems of poor solubility of minodronic acid crystal form I, unfavorable industrial production, low bioavailability and the like, and achieves less solvent usage, low cost, Prepare simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of minodronic acid crystal form II
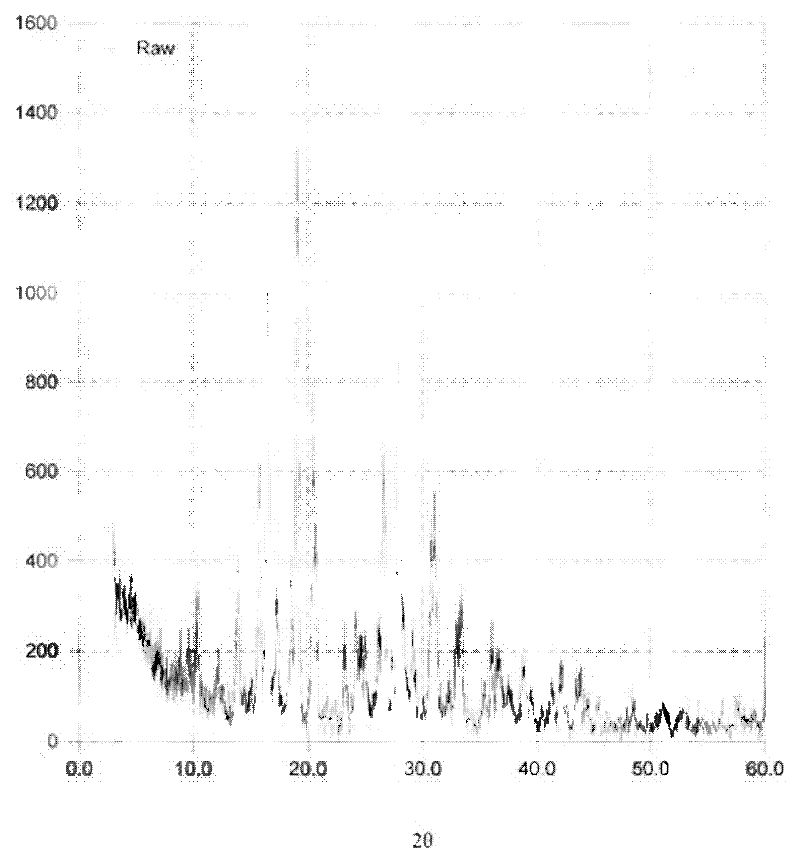
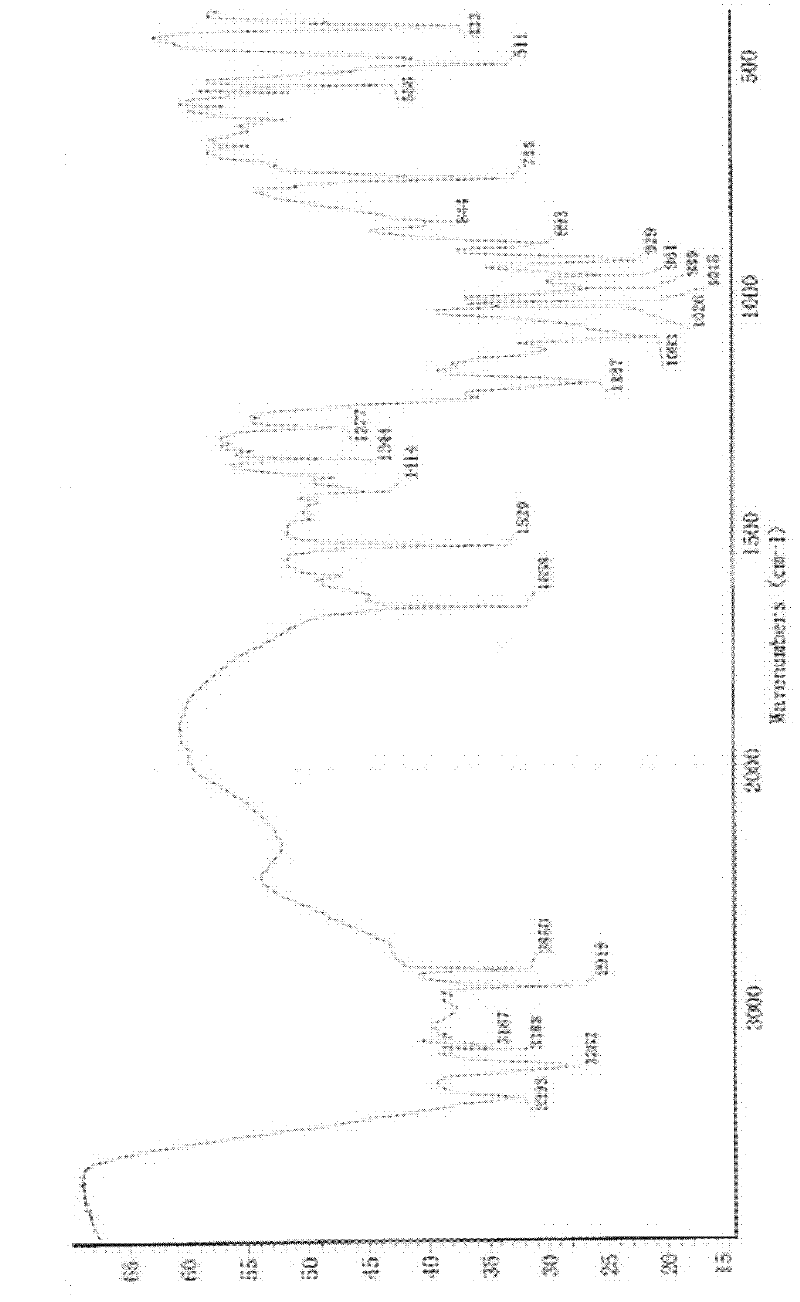
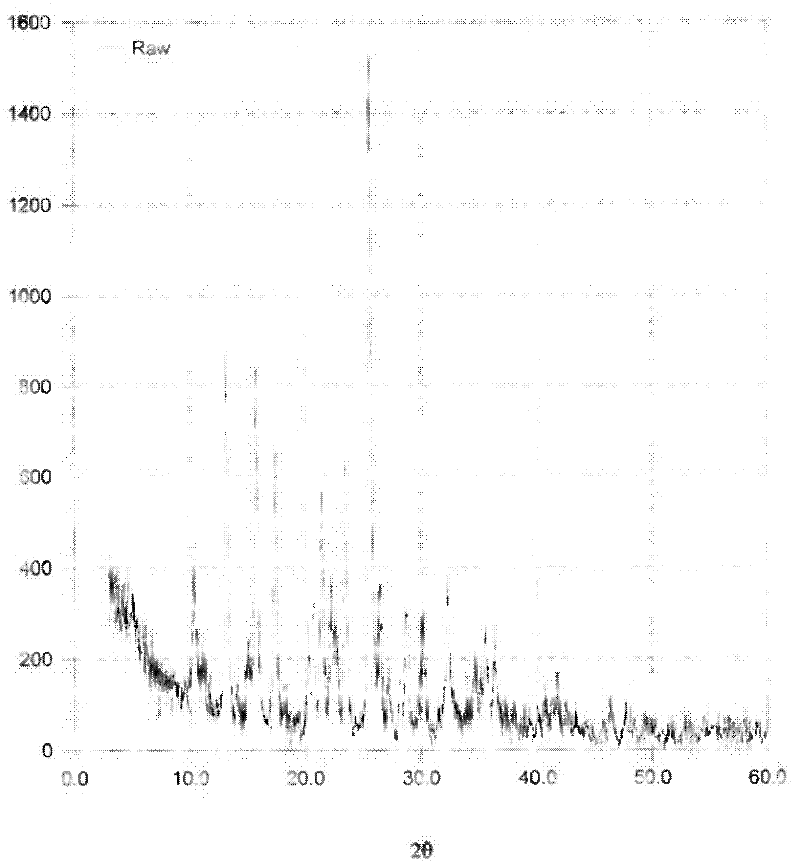
[0045] Add 2g of minodronic acid crude product and 6ml of 6mol / L hydrochloric acid into the three-neck flask, raise the temperature to 60°C, stir to completely dissolve, slowly add methanol dropwise, and keep the reflux state, stop adding methanol dropwise when there is a little turbidity, and cool down Stir at 0°C for 2 hours, let cool to crystallize, filter, and blow dry at 80°C to obtain 1.72 g of Form II sample. Its X-ray diffraction pattern is shown in image 3 , see the infrared spectrum Figure 4 .
Embodiment 2
[0047] Minodronic Acid Form II Tablets
[0048] Tablet prescription:
[0049] Name of raw material
Dosage
Minodronic acid crystalline form II
1.0g
36.0g
66.0g
Crospovidone
6.0g
1.0g
water
about 40ml
Made into 1000 pieces
[0050] Coating Solution Prescription:
[0051] Name of raw material
Dosage
Opadry 81W680000
36.0g
water
200ml
[0052] Tablet core preparation process:
[0053] Pass the raw and auxiliary materials through 80-mesh sieves for later use; mix the main ingredient with crospovidone, microcrystalline cellulose, and lactose evenly in equal increments; use water as a wetting agent to make soft materials from the above-mentioned mixed fine powder, and pass through Granulate with a 24-mesh sieve, dry at 60°C for about 2 hours, control the water content of the g...
Embodiment 3
[0057] Minodronic Acid Form II Capsules
[0058] prescription:
[0059] Name of raw material
Dosage
Minodronic acid crystalline form II
1.0g
36.0g
66.0g
1.0g
water
about 40ml
Make 1000 capsules
[0060] Preparation Process:
[0061] Pass the raw and auxiliary materials through 80-mesh sieve for later use; mix the main ingredient, starch, and lactose evenly in equal increments; use water as a wetting agent to make soft materials from the mixed fine powder, granulate through a 24-mesh sieve, and dry at 60°C About 2 hours, control the water content of the granules to ≤3%, sieve the granules with 24 mesh, and mix well; take the above granules, add the prescribed amount of magnesium stearate, mix well, measure the content of intermediates, fill in No. 4 capsules, and you are done. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com