A kind of solid lipid nanoparticle containing andrographolide and its preparation method and application
A technology of solid lipid nanometer and andrographolide, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, cardiovascular system diseases, etc., can solve the problems of no solid lipid nanoparticles, etc., to improve the degree of dispersion and Effects of bioavailability, increased targeting, and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1) Take 45mg of andrographolide monomer, 250mg of glyceryl stearate, 250mg of glyceryl behenate, and 800mg of lecithin in a wedge-shaped bottle, add an appropriate amount of absolute ethanol, and dissolve it completely in a water bath at 80°C , the organic phase was obtained, the solvent was evaporated to dryness, cooled in an ice bath to form a solid, and set aside;
[0047] 2) Dissolve 1.5g Tween-80 in 50ml pure water to form a uniform water phase;
[0048] 3) mixing the solid prepared above with the water phase, stirring at a high speed of 10,000 rpm, so that the solid is uniformly dispersed to obtain colostrum;
[0049] 4) Perform high-pressure homogenization treatment with 60 MPa pressure at room temperature for 8 to 10 times, and then rapidly cool in an ice bath to obtain solid lipid nanoparticles containing andrographolide.
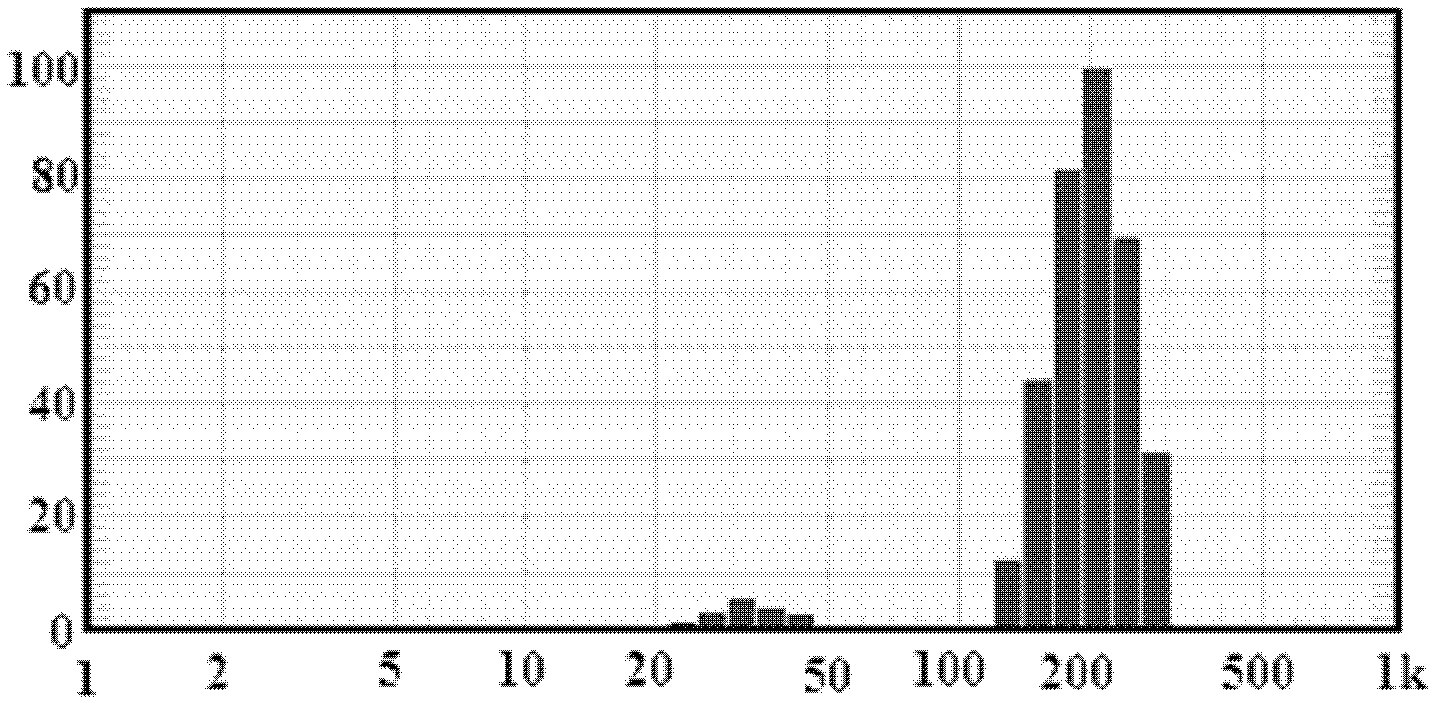
[0050] figure 1 For the particle size distribution figure of the prepared solid lipid nanoparticles containing andrographolide, by figur...
Embodiment 2
[0064] The solid lipid nanoparticles containing neoandrographolide monomer were prepared by the same method as in Example 1, wherein 45 mg of andrographolide monomer was replaced by 45 mg of neoandrographolide monomer.
[0065] It is determined that: the encapsulation efficiency of the solid lipid nanoparticles containing neoandrographolide monomer prepared in this embodiment is 91.2%, the drug loading is 3.52%, the average particle diameter is 256.4nm, and the average zeta potential is -19.8mv.
Embodiment 3
[0067] The same method as in Example 1 was used to prepare solid lipid nanoparticles containing dehydroandrographolide monomer, wherein 45 mg of andrographolide monomer was replaced by 45 mg of dehydroandrographolide monomer.
[0068] It is determined that: the encapsulation efficiency of the solid lipid nanoparticles containing dehydroandrographolide monomer prepared in this embodiment is 90.8%, the drug loading is 3.28%, the average particle diameter is 221.7nm, and the average zeta potential is -19.5mv.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com