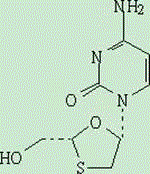
Lamivudine preparation and preparation method thereof
A technology for lamivudine and preparations, which is applied in pharmaceutical formulations, antiviral agents, pill delivery, etc., can solve the problems of low initial dissolution rate, large difference in loading amount, poor content uniformity, etc., and achieves a simple and easy preparation method The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
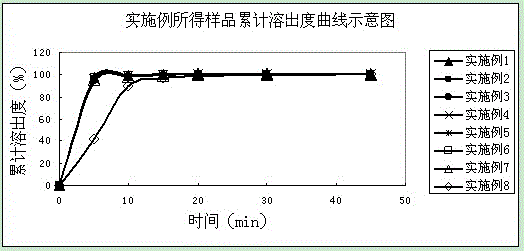
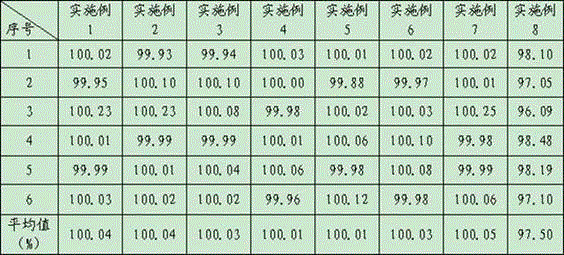
Examples
Embodiment 1
1g
[0027] Preparation:
[0028] (1) Separately sieve lamivudine, lactose, microcrystalline cellulose, and sodium carboxymethyl starch, and mix well to obtain a mixed powder;
[0029] (2) Add 2.5% povidone ethanol solution into the mixed powder to make soft material, granulate, granulate after drying, add micropowder silica gel and magnesium stearate, and mix well;
[0030] (3) Packed into capsules or compressed tablets;
Embodiment 2
[0032] components
[0033] Preparation method is the same as embodiment 1
Embodiment 3
[0035] components
[0036] Preparation method is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com