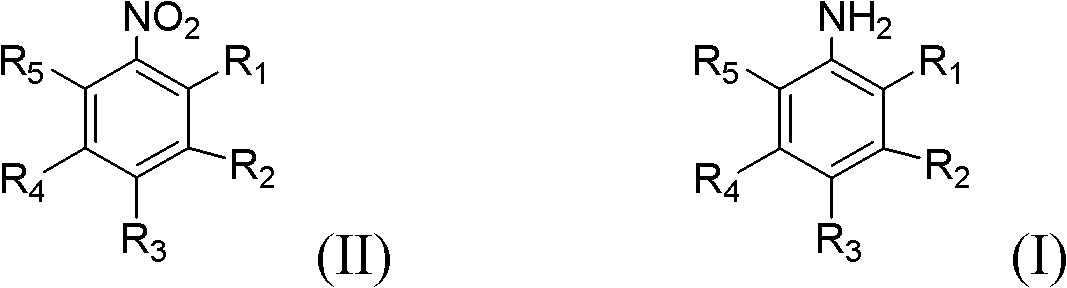A method for preparing aromatic amines by catalytic hydrogenation of aromatic nitro compounds
An aromatic nitro, catalytic hydrogenation technology, applied in the preparation of amino compounds, the preparation of amino hydroxy compounds, the preparation of organic compounds, etc., can solve problems such as insignificant effects, and achieve a wide range of applications, reduced content, and simple use methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 5
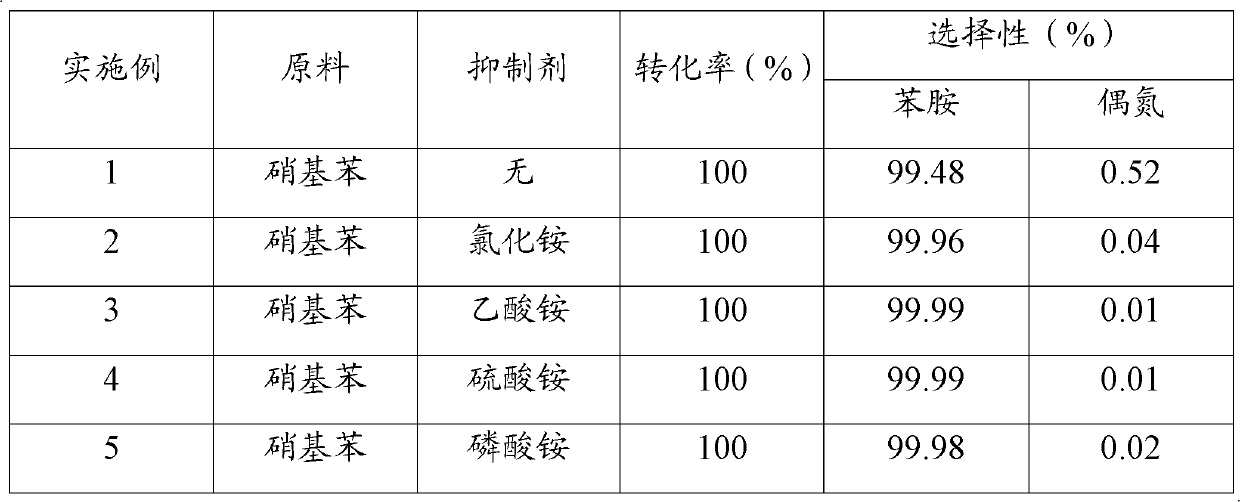
[0026] Examples 1 to 5 investigated the influence of inhibitor types on the inhibitory effect of azo compounds. In a 500ml stainless steel reaction kettle, add 100ml nitrobenzene, 200ml ethanol, 0.5g 5% Pd / C catalyst (produced by Degong Chemical Co., Ltd., Deqing County, Zhejiang Province), no inhibitor or 0.01g inhibitor, close the reaction kettle , Replace the air in the reactor with nitrogen three times, and then replace it with hydrogen three times; raise the temperature to 60°C, hydrogen pressure to 2MPa, start stirring at a stirring rate of 900r / min, and react for 1h; stop the reaction, and wait until the temperature drops to room temperature , The reaction solution was taken out, the catalyst was removed by filtration, and the filtrate was analyzed by gas chromatography. The experimental results are shown in Table 1.
[0027] Table 1 Inhibitor Types Influence on the Inhibitory Effect of Azo Compounds
[0028]
Embodiment 6 10
[0030] Embodiments 6 to 10 investigated the influence of inhibitor dosage on the inhibitory effect of azo compounds. In a 500ml stainless steel reaction kettle, add 100ml o-nitrotoluene, 200ml methanol, 2g Raney-Ni catalyst (produced by Zhejiang Kony Alloy Manufacturing Co., Ltd.), add different amounts of inhibitors, close the reaction kettle, and replace the inside of the reaction kettle with nitrogen. The air was replaced three times with hydrogen; the temperature was raised to 70°C, the hydrogen pressure was 1MPa, stirring was started, the stirring rate was 900r / min, and the reaction was 1.5h; the reaction was stopped, and after the temperature dropped to room temperature, the reaction solution was taken out and filtered The catalyst was removed and the filtrate was analyzed by gas chromatography. The experimental results are shown in Table 2.
[0031] Table 2 Influence of inhibitor dosage on the inhibitory effect of azo compounds
[0032]
Embodiment 11 2
[0034] Examples 6 to 10 investigated the influence of inhibitors on the inhibitory effect of azo compounds when different aromatic nitro compounds were used as raw materials. In a 500ml stainless steel reactor, add 100ml of aromatic nitro compounds, 200ml of ethanol, 1g of 5% Pt / C catalyst (produced by Degong Chemical Co., Ltd., Deqing County, Zhejiang Province), add inhibitors, close the reactor, and replace the reactor with nitrogen The air was replaced three times with hydrogen; the temperature was raised to 100°C, the hydrogen pressure was 1.5MPa, stirring was started, the stirring rate was 900r / min, and the reaction was 2h; the reaction was stopped, and after the temperature dropped to room temperature, the reaction solution was taken out and filtered The catalyst was removed and the filtrate was analyzed by gas chromatography. The experimental results are shown in Table 3.
[0035] The influence of table 3 inhibitor on the inhibitory effect of different aromatic nitro com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com