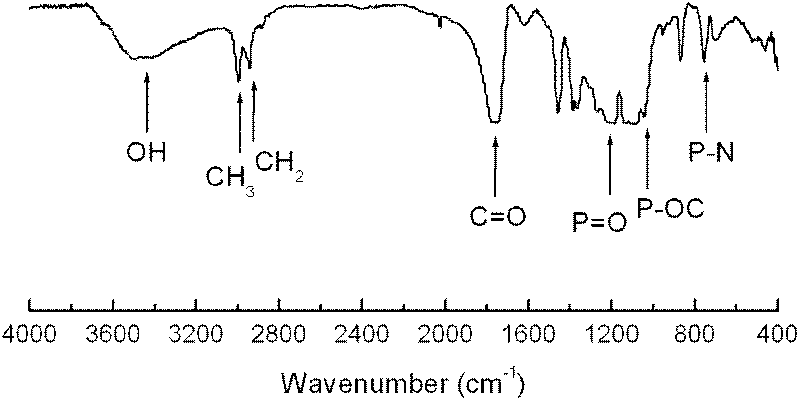
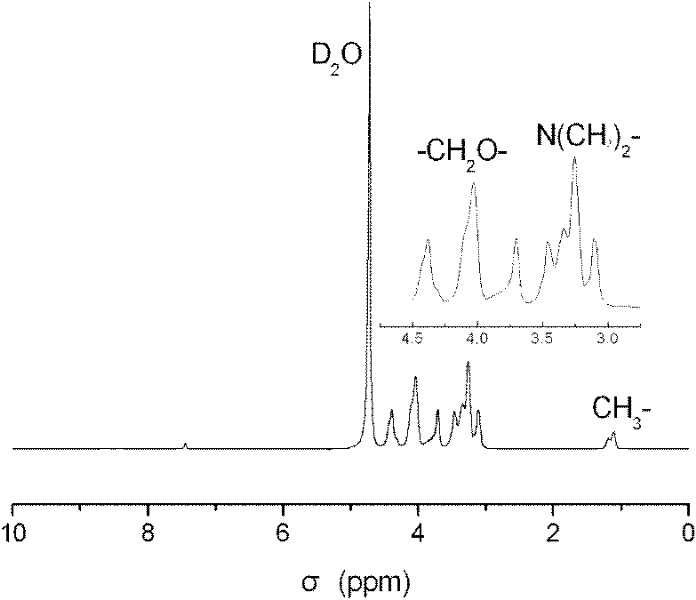
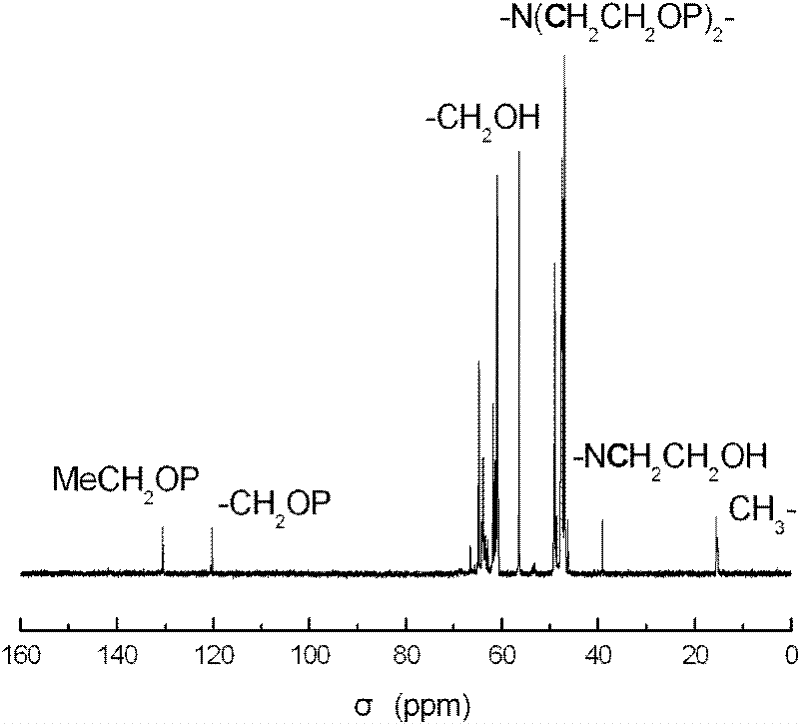
A kind of hyperbranched polyphosphoramidite and preparation method thereof
A technology of polyphosphoramidate and hyperbranched polymers, which is applied in the direction of pharmaceutical formulations and medical preparations of non-active ingredients, etc. It can solve the problems of limiting the application of phospholipid polymers and the inability of phospholipid bonds to be degraded by polymers.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Example 1: 0.1 mol of diisopropanolamine and 0.4 mol of triethylamine were dissolved in 50 mL of dichloromethane and 5 mL of DMF, and 0.15 mol of phenyl dichlorophosphate was added thereto under stirring. Reacted at 30°C for 24h, then warmed to 60°C and reacted at this temperature for 24h. After the reaction, the flask was cooled and washed with 2mol / L hydrochloric acid and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated to the minimum volume under reduced pressure, precipitated with dichloromethane-anhydrous ether three times, and dried in vacuo at 60°C for 24 hours to obtain a pale yellow viscous target polymer. The molecular weight of the product is 6500.
example 2
[0029] Example 2: 0.1 mol of dipropanolamine and 0.4 mol of triethylamine were dissolved in 50 mL of dichloromethane and 5 mL of DMF, and 0.15 mol of phenyl dichlorophosphate was added thereto under stirring. Reacted at room temperature for 24h, then warmed to 60°C and reacted at this temperature for 24h. After the reaction, the flask was cooled and washed with 2mol / L hydrochloric acid and saturated sodium chloride solution, dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated to the minimum volume under reduced pressure, precipitated with dichloromethane-anhydrous ether three times, and dried in vacuo at 60°C for 24 hours to obtain a pale yellow viscous target polymer. The molecular weight of the product is 9500.
example 3
[0030] Example 3: 0.10 mol of dipropanolamine and 0.40 mol of N,N-dimethylaniline were dissolved in 20 mL of tetrahydrofuran and 50 mL of toluene, and 0.15 mol of methyl dichlorophosphate was added thereto under stirring. Reacted at 30°C for 24h, then warmed to 80°C and reacted at this temperature for 48h. After the reaction was completed, the flask was cooled, and the supernatant liquid was poured out. The residual substance was dissolved in chloroform and precipitated in anhydrous ether. The above solvent-precipitation process was repeated three times, and after filtration, washing, and vacuum drying, a white solid was obtained, which was the target polymer. The molecular weight of the product is 27600.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com