Bufalin-loaded cyclic peptide-modified polyethylene glycol-polylactic acid hydroxyl glycolic acid-polylysine nanoparticles
A technology of poly(lactic-co-glycolic acid) and poly-lysine, which is applied in the direction of medical preparations containing active ingredients, organic active ingredients, non-active ingredients of polymer compounds, etc., and can solve problems such as low solubility, limited application, and toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
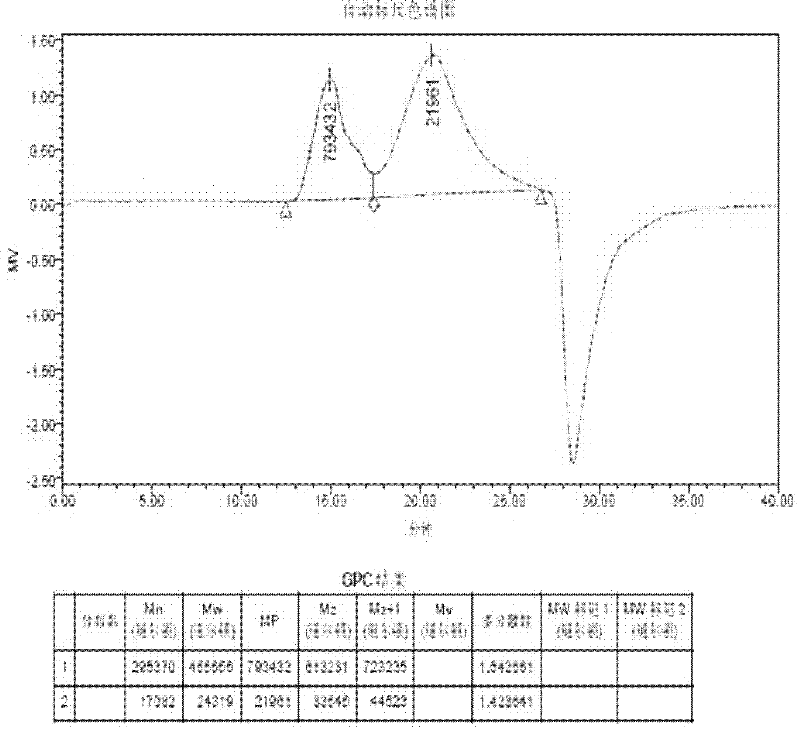
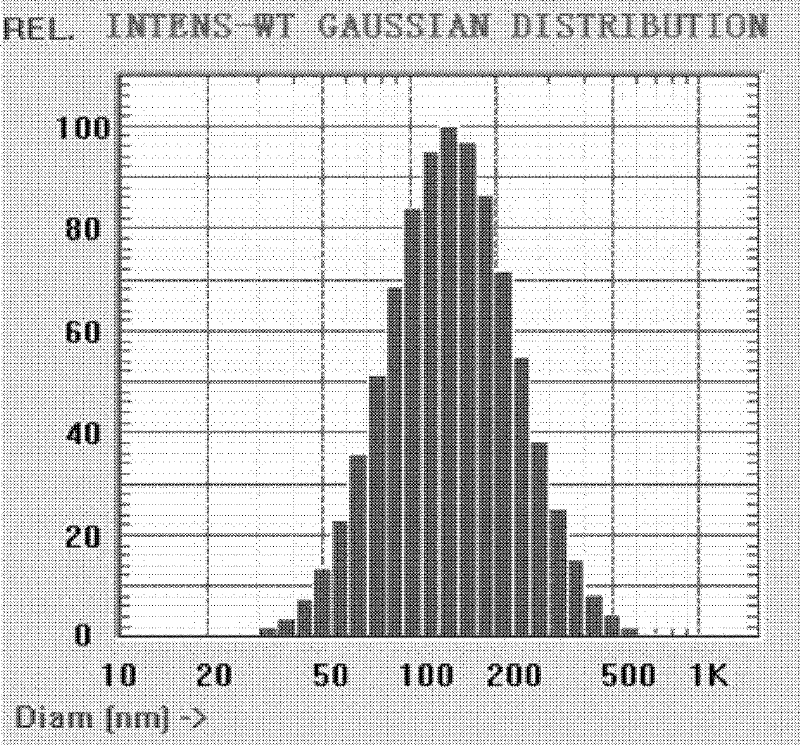
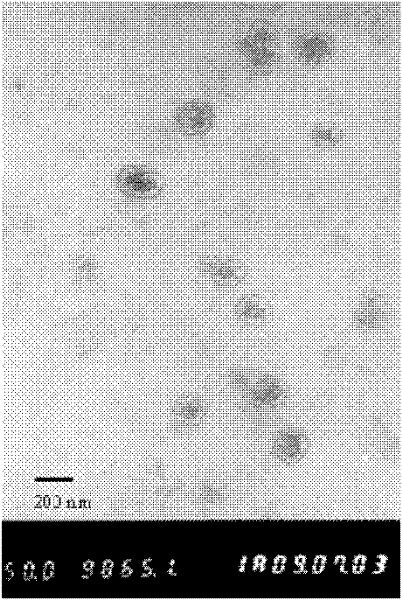
Image
Examples
Embodiment 1
[0054] Example 1. Valine-arginine-glycine-aspartate-glutamate cyclic peptide (VRGDG, cRGD) modified polyethylene glycol monomethyl ether-polylactic acid-glycolic acid-polylysine ( mPEG-PLGA-PLL-cRGD) preparation
[0055] Preparation of mPEG-PLGA: heat and dry the heat-resistant glass tube under vacuum, add lactide and glycolide raw materials in a certain molar mass ratio (ratio 8: 2, 7: 3 or 5; 5), add the total amount of raw materials PEG with a mass percentage of 1%-15% and a molecular weight range of 350-5000 is added to the catalyst, nitrogen is passed through, heated to dissolve, vacuumed, cooled and solidified, vacuumed for 2 hours, then sealed, and reacted at 120-150°C for 8-50 hours. (2) Preparation of mPEG-PLGA-Boc(Z): A certain amount of mPEG-PLGA is dissolved in a dry organic solvent, and Boc-Phe (1-15eqv.), N,N-dicyclohexylcarbodiimide (1 ~15eqv.), slowly drip 4-dimethylaminopyridine at 0~40℃, protected by nitrogen, stirred at room temperature for 1~3 days, filtered,...
Embodiment 2
[0057] Example 2. Preparation of mPEG-PLGA-PLL-cRGD nanoparticles containing bufalin drug
[0058] Prepared by double emulsion method, dissolve 4mg or 20mg material mPEG-PLGA-PLL-cRGD in 200μL or 1000μL dichloromethane or a mixed solvent of dichloromethane and acetone, add 0.2mg or 4mg bufalin drug solution, phacoemulsify (300W Or 500W, 10s×4), then add 2.2mL or 6mL of Pluronic F68 aqueous dispersion medium with a concentration of 0.5% or 2%, and then sonicated again (300W or 500W, 10s×4). Then, the organic phase is removed by stirring for 0.5-5h at room temperature, and a nanoparticle solution with a particle size of 100-600nm is obtained.
[0059] Prepared by thin film emulsification method, take 4mg or 20mg material mPEG-PLGA-PLL-cRGD and 0.2mg or 4mg bufalin bufalin drug dissolved in 400μL or 2000μL acetone solvent, rotary evaporation to form a film, then add 4mL or 12mL of aqueous solution, at room temperature Stir for 0.5-6h to obtain a nanoparticle solution with a particle ...
Embodiment 3
[0065] Example 3. Toxicity of mPEG-PLGA-PLL-cRGD nanoparticles (with a particle size of 100-400 nm) to SW620 intestinal cancer cells
[0066] Observe the survival rate of SW620 cells by CKK-8 colorimetry, from Figure 4 It can be seen that as the concentration increases, the cell survival rate of mPEG-PLGA-PLL and mPEG-PLGA-PLL-cRGD blank nanoparticles is between 90% and 96%, with no significant change, indicating that they are basically non-toxic. Compared with Bufalin, the cytotoxicity of Bufalin nanoparticles was significantly increased, indicating that Bufalin-mPEG-PLGA-PLL and Bufalin-mPEG-PLGA-PLL-cRGD drug-loaded nanoparticles can effectively increase the cytotoxicity of Bufalin, which will help Improve the effect of tumor treatment.
[0067] Implementation column 4, mPEG-PLGA-PLL-cRGD nanoparticles (particle size 100-300nm)
[0068] Targeted imaging of nude mice bearing SW620 bowel cancer
[0069] Rhodamine B (Rb) is used as a fluorescent probe to be encapsulated in nanoparti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com