Chelate type ionic liquid and preparation and purification method thereof
A technology of ionic liquid and purification method, which is applied in the field of chelating ionic liquid and its preparation and purification, and can solve the problems of unstable decomposition of carboxylic anions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
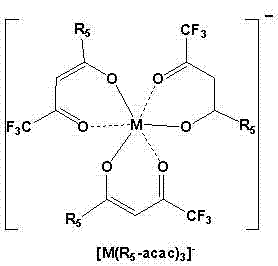
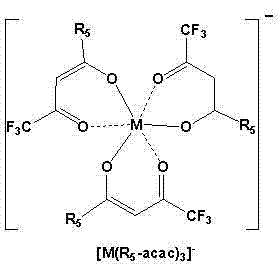
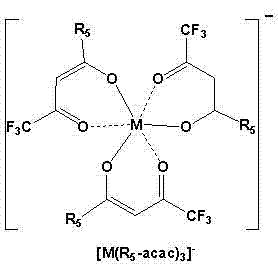
[0040] Add 0.208g, 1mmol of hexafluoroacetylacetone and 15ml of acetonitrile into a 50ml round-bottom single-necked flask, put it in a water bath at 35°C, and then add ammonia water. After 24 hours of reaction, CoCl was added 2 .4H 2 O 0.33mmol, carry out again 8 hours, add trimethyl n-hexadecyl ammonium bromide 0.33mmol, react and carry out 10 hours again, filter and remove inorganic salt (NH 4 Br, NH 4 Cl), the acetonitrile was evaporated by a rotary evaporator to obtain a red liquid, which became solid after cooling.
[0041] Dissolve the obtained crude product in 10 mL of ethyl acetate, then add an equal volume of water, shake for 3 minutes, and separate into layers. The upper layer is the ionic liquid phase, and the lower layer is the water phase. Separation to remove the water layer, repeat the above steps 3 times , the rich ionic liquid phase was collected, and the solvent was removed by a rotary evaporator, and the resulting product was vacuum-dried for 24 hours to ...
Embodiment 2
[0043] Add 0.208g, 1mmol of hexafluoroacetylacetone and 15ml of acetonitrile into a 50ml round-bottom single-necked flask, put it in a water bath at 35°C, and then add ammonia water. After 24 hours of reaction, CoCl was added 2 .4H 2 O 0.33mmol, carry out again 8 hours, add tetrabutylammonium chloride 0.33mmol, react and carry out 10 hours again, filter and remove inorganic salt (NH 4 Cl), the acetonitrile was evaporated by a rotary evaporator to obtain a red liquid, which became solid after cooling.
[0044] Dissolve the obtained crude product in 10 mL of ethyl acetate, then add an equal volume of water, shake for 3 minutes, and separate into layers. The upper layer is the ionic liquid phase, and the lower layer is the water phase. Separation to remove the water layer, repeat the above steps 3 times , the rich ionic liquid phase was collected, and the solvent was removed by a rotary evaporator, and the resulting product was vacuum-dried for 24 hours to obtain purified [(C ...
Embodiment 3
[0046] Add 0.208g, 1mmol of hexafluoroacetylacetone and 15ml of acetonitrile into a 50ml round-bottom single-necked flask, put it in a water bath at 35°C, and then add ammonia water. After 24 hours of reaction, CoCl was added 2 .4H 2 O 0.33mmol, carry out 8 hours again, add triphenyl n-butyl phosphine chloride 0.33mmol, react and carry out 10 hours again, filter and remove inorganic salt (NH 4 Cl), the acetonitrile was evaporated by a rotary evaporator to obtain a red liquid, which became solid after cooling.
[0047] Dissolve the obtained crude product in 10 mL of ethyl acetate, then add an equal volume of water, shake for 3 minutes, and separate into layers. The upper layer is the ionic liquid phase, and the lower layer is the water phase. Separation to remove the water layer, repeat the above steps 3 times , the rich ionic liquid phase was collected, and the solvent was removed by a rotary evaporator, and the obtained product was vacuum-dried for 24 hours to obtain purifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com