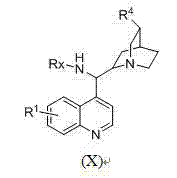
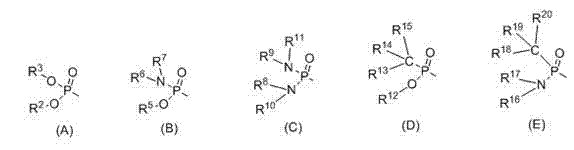
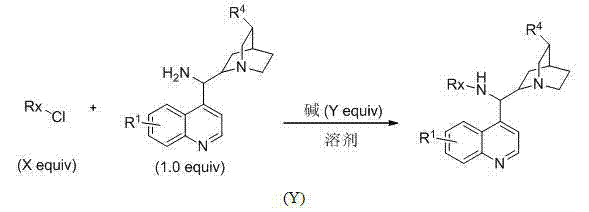
Phosphoric acid amide bifunctional catalyst and synthetic method thereof
A dual-functional catalyst and synthesis method technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., to achieve cheap raw materials, good yields, and high catalytic efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: preparation catalyst
[0049]
[0050] Under nitrogen protection, into a 50 ml round bottom flask was added cinchonidine-derived primary amine (1.0 mmol, 293 mg) and anhydrous CH 2 Cl 2 , and triethylamine (3.0 mmol, 303 mg) was added after complete dissolution. Diethoxyphosphoryl chloride (1.2 mmol, 206.4 mg) was added dropwise at 0 °C, stirred overnight and post-treated: adding 30 ml of water, extracted with dichloromethane, dried over anhydrous sodium sulfate, and column chromatographed to obtain Pale yellow oily pure product catalyst, its structure is shown in the above reaction formula, and the productive rate is 58%. 1 H NMR (400 MHz, CDCl 3 ): 8.68 (s, 1H), 8.02-7.82 (m, 2H), 7.68-7.28 (m, 3H), 5.72-5.68 (m, 1H), 5.02-4.97 (m, 2H), 4.20-3.82 (m, 1H ), 2.24-2.13 (m, 1H), 1.52-1.11 (m, 7H); 13 C NMR (100 MHz, CDCl 3 ): 150.6, 147.9, 141.7, 129.5, 128.5, 126.9, 125.8, 123.5, 121.3, 114.4, 77.8, 76.8, 63.7, 59.6, 54.5, 42.7, 33.2, 29.3, 14...
Embodiment 2
[0051] Embodiment 2: preparation catalyst
[0052]
[0053] Into a 50-ml round-bottomed flask under nitrogen protection, add a Cinchoni-derived primary amine (1.0 mmol, 323 mg) and anhydrous CH 2 Cl 2 , and triethylamine (3.0 mmol, 303 mg) was added after complete dissolution. Diethoxyphosphoryl chloride (1.2 mmol, 206.4 mg) was added dropwise at 0 °C, stirred overnight and post-treated: add 30 ml of water, extract with dichloromethane, dry over anhydrous sodium sulfate and perform column chromatography to obtain Pure product catalyst, its structure is shown in above reaction formula, productive rate 62%. 1 H NMR (400 MHz, CDCl 3 ): 8.68 (s, 1H), 8.02-7.82 (m, 2H), 7.68-7.28 (m, 3H), 5.72-5.68 (m, 1H), 5.02-4.97 (m, 2H), 4.20-3.82 (m, 1H ), 2.35 (m, 3H), 2.24-2.13 (m, 1H), 1.52-1.11 (m, 7H); 13 C NMR (100 MHz, CDCl 3 ): 150.6, 147.9, 147.7, 129.5, 128.5, 126.9, 125.8, 123.5, 121.3, 114.4, 77.8, 76.8, 63.7, 59.6, 54.5, 42.7, 33.2, 29.3, 21.3, 14.1, 14.0. MS (EI):...
Embodiment 3
[0054] Embodiment 3: preparation catalyst
[0055]
[0056] Under nitrogen protection, add binaphthol (1.0 mmol, 186 mg), anhydrous CH 2 Cl 2 and triethylamine (3.0 mmol, 303 mg), then dropwise added phosphorus oxychloride (1.0 mmol, 151 mg) under ice-cooling, gradually warmed up to room temperature, stirred at room temperature for 3 hours, then added dropwise the prepared Cenco Nidine-derived primary amine (1.0 mmol, 294 mg) and anhydrous CH 2 Cl 2 After stirring the mixed solution overnight, carry out post-treatment: add 30 ml of water, extract with dichloromethane, dry over anhydrous sodium sulfate and perform column chromatography to get the pure product catalyst, its structure is shown in the above reaction formula, and the yield is 55 %. 1 H NMR (400 MHz, CDCl 3 ): 8.66 (s, 1H), 8.02-7.31 (m, 6H), 7.04-6.79 (m, 7H), 5.07-4.97 (m, 2H), 4.15-4.10 (m, 1H), 2.85-2.00 (m, 7H ), 1.42-1.41 (m, 3H); 13 C NMR (100 MHz, CDCl 3 ): 156.3, 156.2, 150.6, 147.9, 141.7, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com