Preparation method for 2,2',4,4'-tetrehydroxybenzophenone
A technology of tetrahydroxybenzophenone and dihydroxybenzoic acid is applied in the field of preparation of 2,2',4,4'-tetrahydroxybenzophenone, and can solve the problems of high unit consumption of raw materials, complex by-products, and reaction Difficult to control and other problems, to achieve the effect of reducing production energy consumption, shortening process time, and accelerating catalytic speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
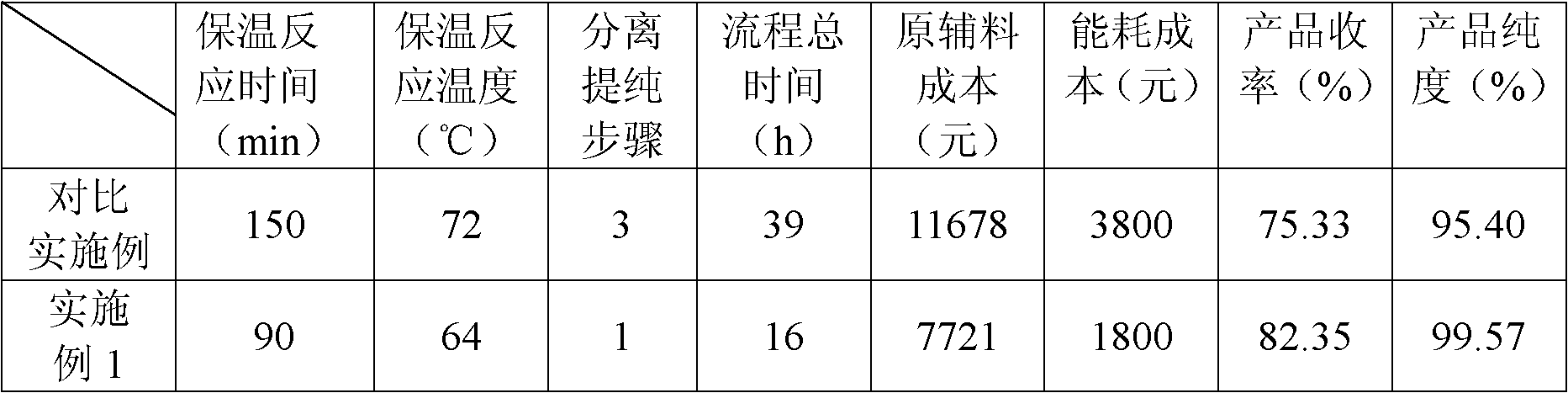
[0023] Drop into 54kg phosphorus oxychloride, 37.5kg sulfolane, 37.5kg dichloroethane, 50kg 2,4-dihydroxybenzoic acid in the reactor, add 4kg phosphorus trichloride, 50kg m-benzene Diphenol and 42kg zinc chloride, start to heat up, and the temperature in the control still is 64 ℃, and insulation reaction makes reaction completely in 90 minutes. After the reaction is over, transfer the product to a kettle with a water temperature below 15°C and a water volume of 1500L to hydrolyze the catalyst. After the hydrolysis is completed, cool down to below 15°C, discharge and filter, then transfer to a kettle with a water volume of 1500L, and put 6kg of activated carbon into it , heated up to 90-100°C, kept stirring for 10 minutes, then discharged and filtered, crystallized, centrifuged, and dried to obtain 65.72 kg of light yellow-green powder.
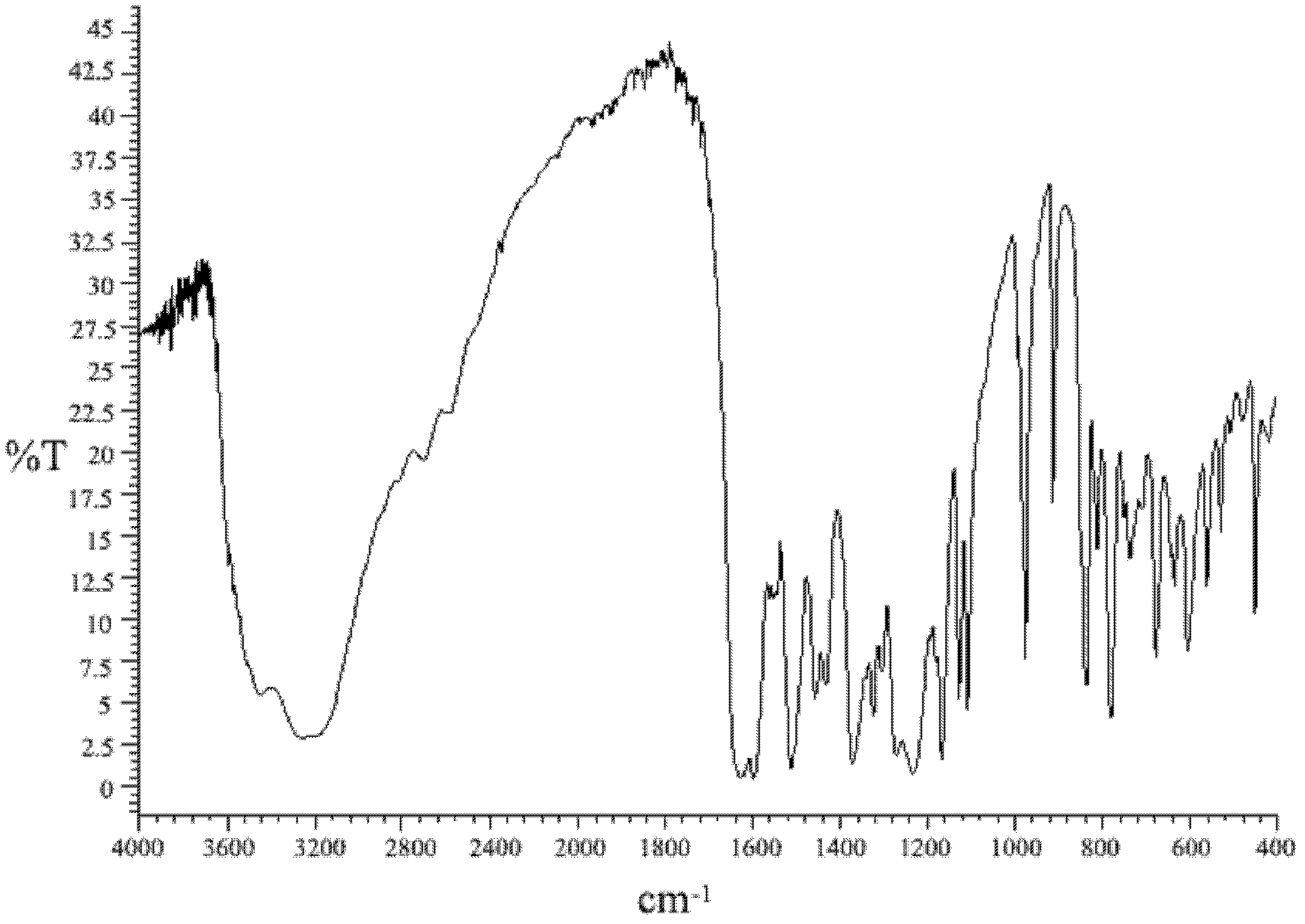
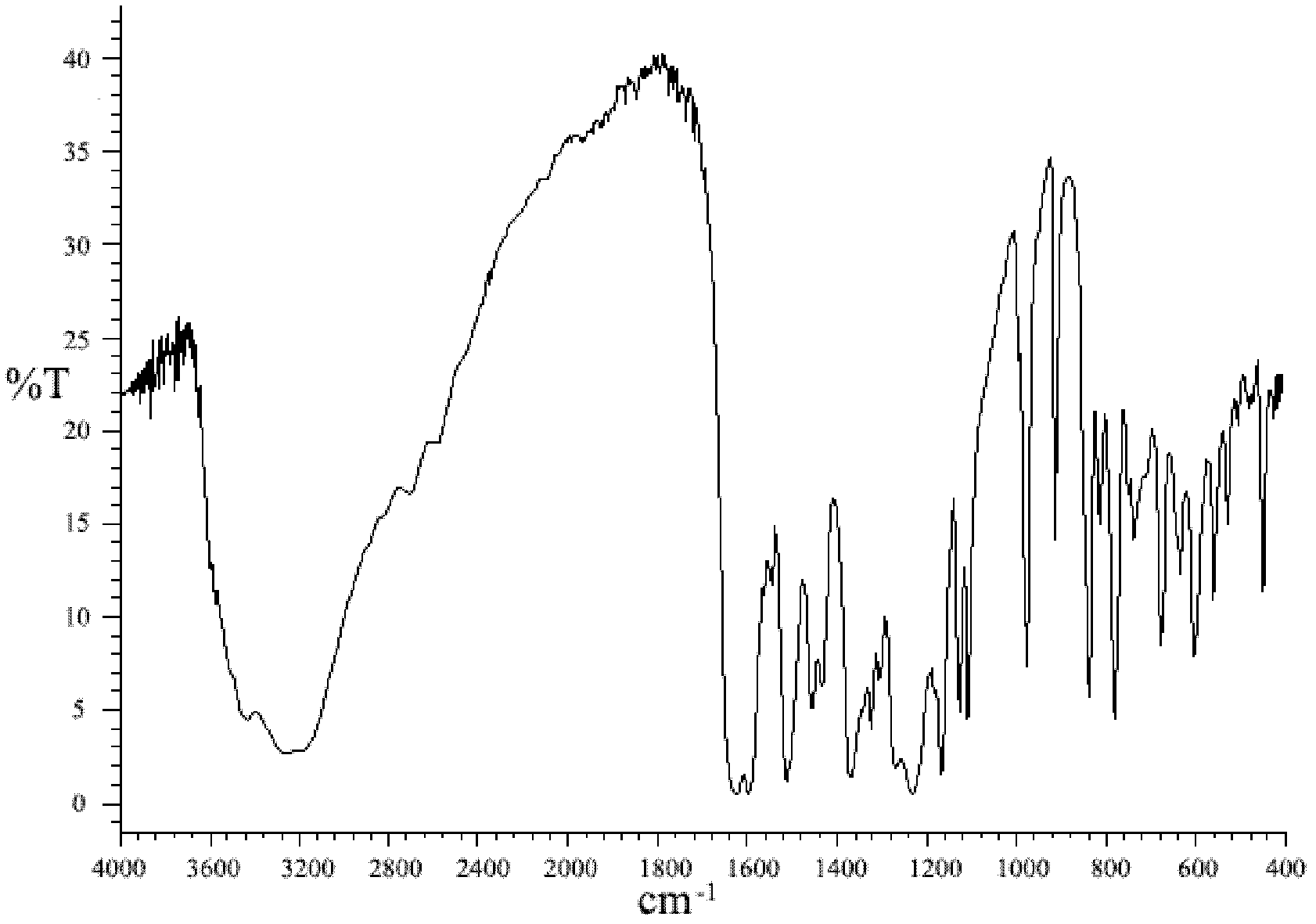
[0024] The final product of this embodiment is characterized by infrared spectroscopy (see figure 1 ), and with the infrared spectrum of 2,2...
Embodiment 2~3
[0030] Keep the feed intake of 50kg 2,4-dihydroxybenzoic acid, 50kg resorcinol, 37.5kg sulfolane, and 37.5kg dichloroethane constant, the mass ratio of catalyst phosphorus oxychloride, zinc chloride, phosphorus trichloride Without changing the total amount of the catalyst, other operating conditions and product characterization methods were the same as in Example 1 to obtain 2,2',4,4'-tetrahydroxybenzophenone. The catalyst dosage and test results are shown in Table 1.
[0031] Table 2. Embodiment 2~3 catalyst total amount and test result
[0032]
Embodiment 4~5
[0034] Keep the feeding amount of 50kg 2,4-dihydroxybenzoic acid, 50kg resorcinol, 54kg phosphorus oxychloride, 42kg zinc chloride, 4kg phosphorus trichloride constant, solvent sulfolane, dichloroethane mass ratio constant , changing the dosage of the solvent, other operating conditions and product characterization methods are the same as in Example 1 to obtain 2,2',4,4'-tetrahydroxybenzophenone. The solvent dosage and test results are shown in Table 3.
[0035] Table 3. Embodiment 4~5 solvent total amount and test result
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com