Method for preparing florfenicol intermediate salt
A technology of florfenicol and intermediates, which is applied in the field of preparation of florfenicol intermediate salts, can solve the problems of large sewage discharge, high cost, difficult recovery of glycerin, etc., and achieves convenient operation, good product quality and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
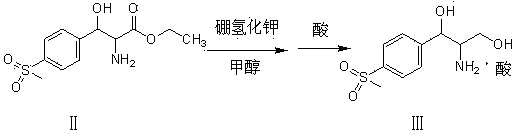
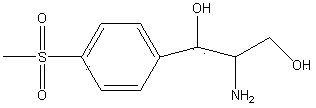
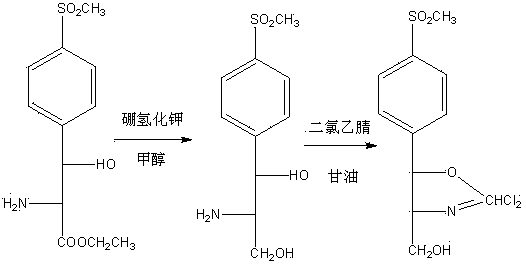
[0022] In the three-necked flask, add 300g (9.36mol) methanol, 50g (0.17mol) D-threo-p-methylsulfonylphenylserine ethyl ester, raise the temperature to 35°C, add 13g (0.24mol) potassium borohydride, and keep warm for 5 hours , then add 50g (0.41mol) concentrated hydrochloric acid to adjust the pH to 3-3.5, keep warm at 75-80°C for 2-2.5 hours, distill off methanol and boron ester, adjust the pH of the residue to 7-7.5 with potassium hydroxide, spray dry, The hydrochloride product of the reduced product of the formula was 49.03 grams, the yield was 100%, the fixed nitrogen content was 4.90%, and the mp (melting point) was 152-154°C.
Embodiment 2
[0024] In the three-necked flask, add 300g (9.36mol) methanol, 50g (0.17mol) D-threo-p-methylsulfonylphenylserine ethyl ester, raise the temperature to 35°C, add 13g (0.24mol) potassium borohydride, and keep warm for 5 hours , then add 30g (0.311mol) of sulfuric acid to adjust the pH to 1-1.5, keep it warm at 50-55°C for 1-1.5 hours, evaporate methanol and boron ester, adjust the pH of the residue to 6-6.5 with sodium hydroxide, and evaporate to dryness under reduced pressure , to obtain 51.22 g of the reduced sulfate product, with a yield of 100%, a fixed nitrogen content of 4.76%, and mp160-163°C.
Embodiment 3
[0026] In the three-necked flask, add 300g (9.36mol) methanol, 50g (0.17mol) D-threo-p-methylsulfonylphenylserine ethyl ester, raise the temperature to 35°C, add 13g (0.24mol) potassium borohydride, and keep warm for 5 hours , and then add 34g (0.301mol) of phosphoric acid to adjust the pH to 1.5-2, keep the temperature at 60-65°C for 3.5-4 hours, evaporate methanol and boron ester, adjust the pH of the residue to 8.5-9.0 with sodium methoxide, and evaporate to dryness under reduced pressure. 50.98 g of the reduced phosphate product was obtained, with a yield of 99.53%, a fixed nitrogen content of 4.74%, and an mp of 155-157°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com