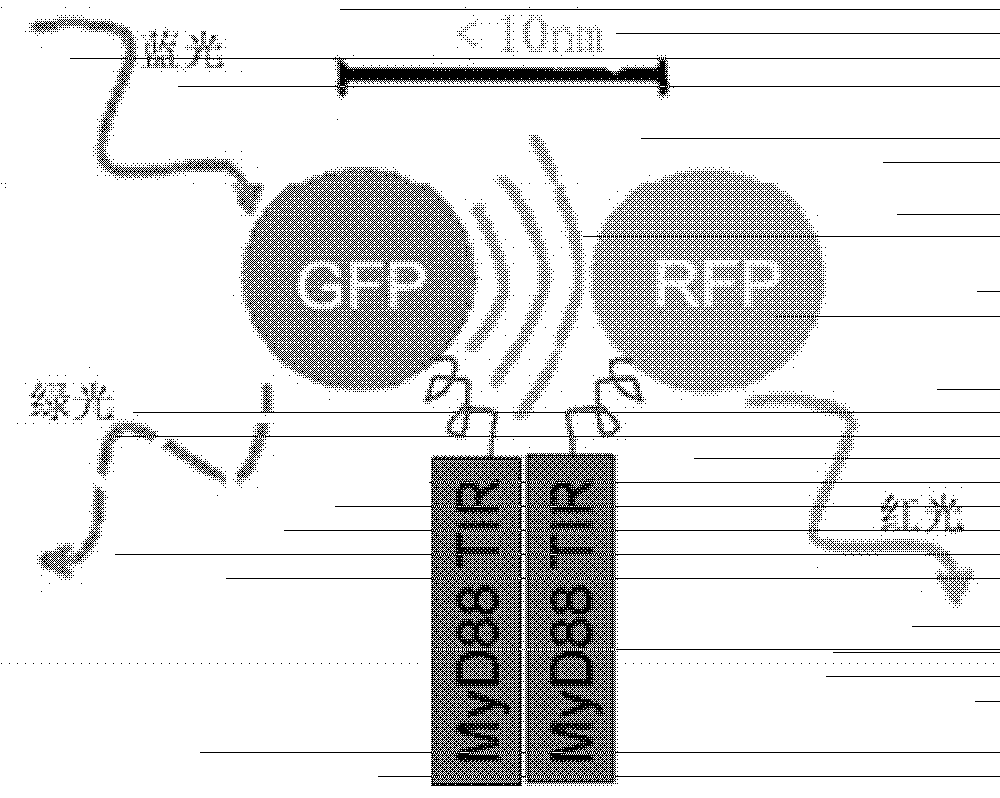Anti-inflammatory inhibitor screening model taking MyD88TIR (myeloid differentiation primary response protein 88 Toll/interleukin-1 receptor) dimerization as target point and application thereof
A technology for inhibitor screening and dimerization, applied in recombinant DNA technology, microbial determination/testing, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
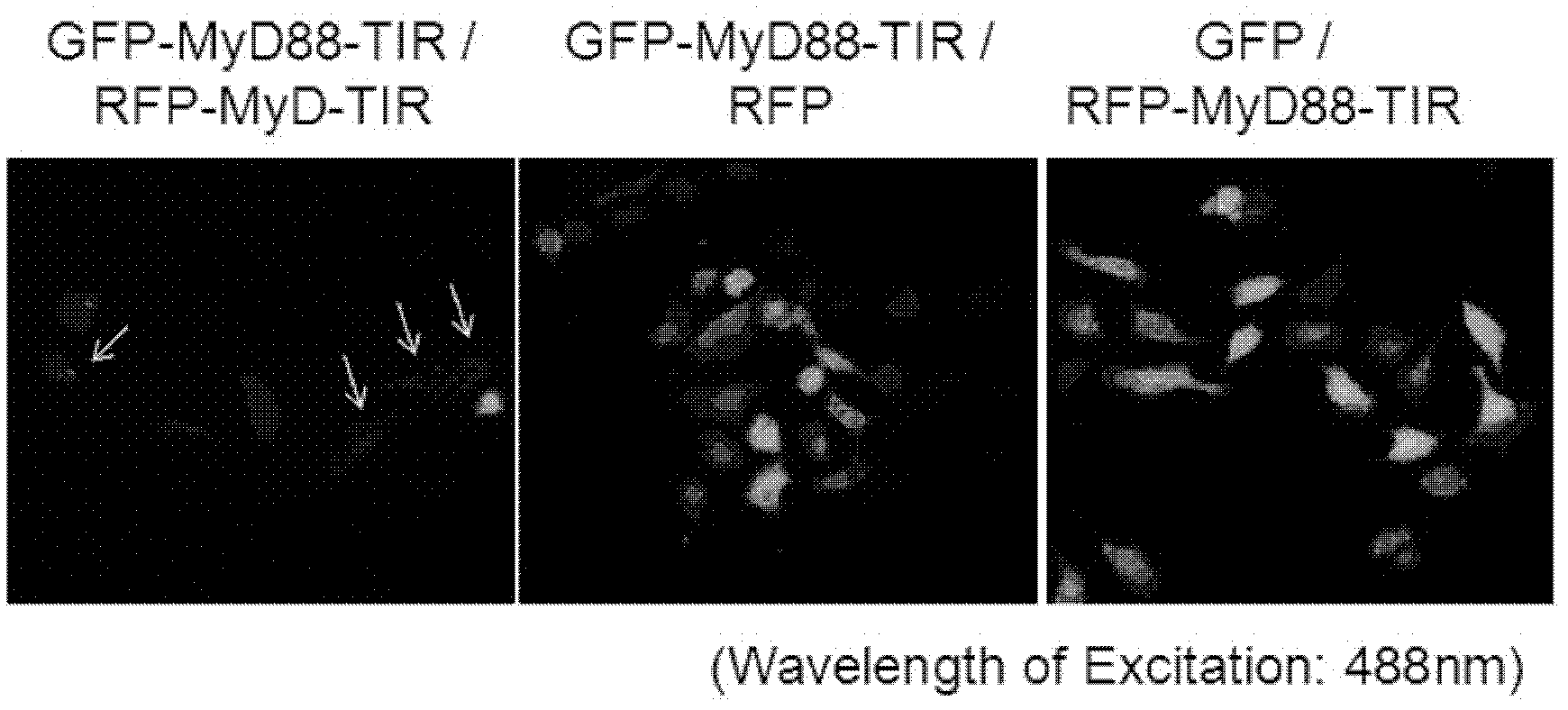
[0042] Example 1: GFP-MyD88 TIR and RFP-MyD88 TIR transfected into mammalian cells can undergo fluorescence resonance energy transfer
[0043] we press figure 1 The combination shown in, the constructed GFP-MyD88 TIR, RFP-MyD88 TIR, GFP, RFP plasmids were co-transfected into HeLa cells. Under 488nm excitation light, cells co-transfected with GFP-MyD88TIR and RFP-MyD88TIR fusion proteins (left photo) emit dim green fluorescence, and some cells even emit orange light. It was proved that the green fluorescence emitted by GFP should be the dimerization of MyD88TIR to transfer the energy to RFP, thus realizing FRET. While the control group was co-transfected with GFP-MyD88 TIR fusion protein and plasmid empty of RFP (middle photo), or conversely, co-transfected with plasmid empty of GFP and RFP-MyD88 TIR fusion protein (right photo), 488nm excitation light Under observation, the cells showed bright green fluorescence. Note: Because the cells co-transfected with GFP-MyD88 TIR and...
Embodiment 2
[0045] Example 2: His-MyD88 TIR recombinant protein can interact with GST-MD88 TIR recombinant protein in vitro
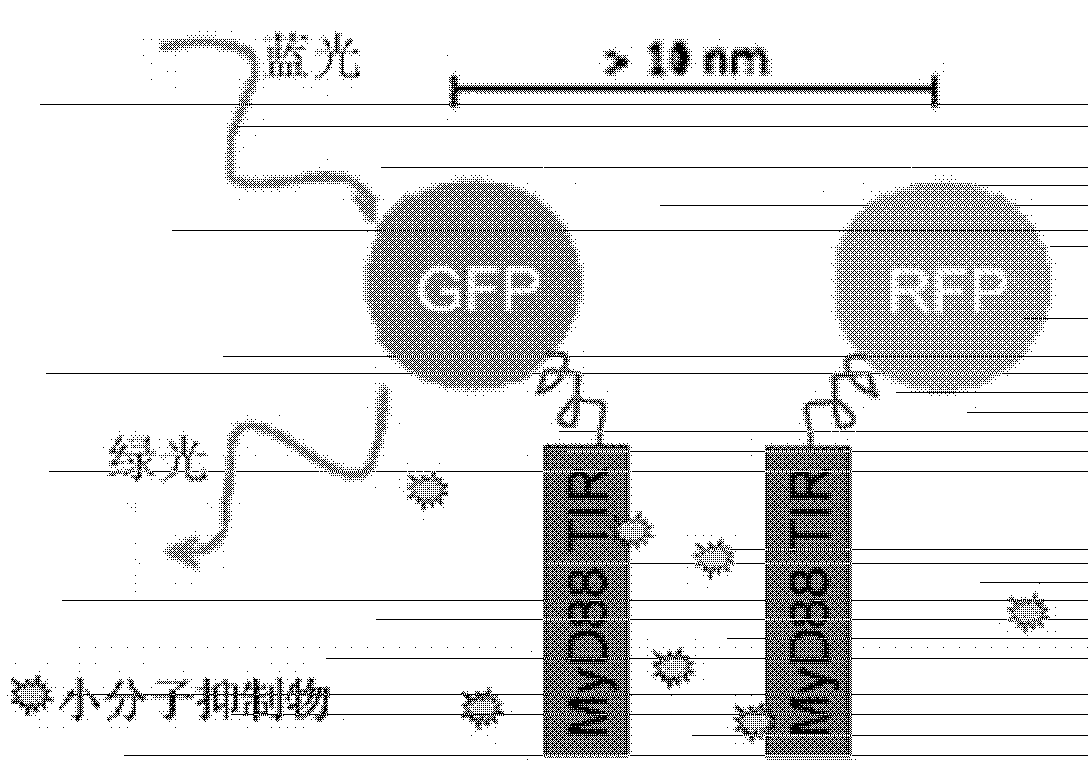
[0046] There is still a problem with the live cell in situ fluorescence model described in Example 1 above: when the FRET phenomenon in cultured cells is blocked by an added compound, we cannot conclude that this compound directly blocks MyD88 TIR The dimerization of MyD88 TIR, or this compound triggers some kind of intracellular signaling cascade that indirectly affects the dimerization of MyD88 TIR. To address this issue, we can use a His-MyD88 TIR and GST-MyD88 TIR recombinant protein interaction assay in vitro to verify.
[0047] To this end, we constructed plasmids for prokaryotic expression of fusion proteins GST-MyD88 TIR and His-MyD88 TIR, and transformed E. coli to induce protein expression (recombinant fusion proteins can be specifically recognized by MyD88 antibody immunoblotting, proving the correct expression of MyD88 TIR) . like Figure 4 As shown,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com