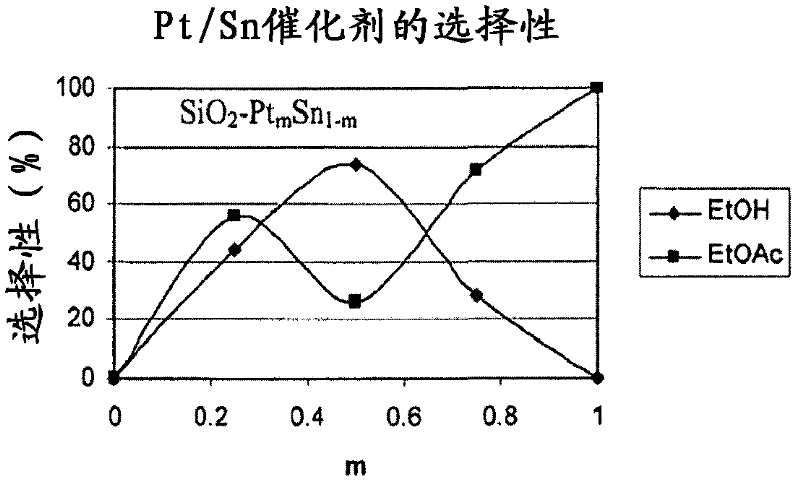
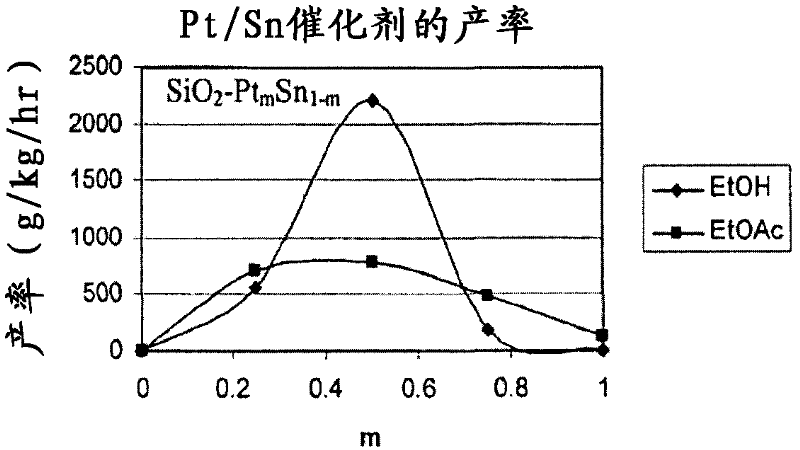
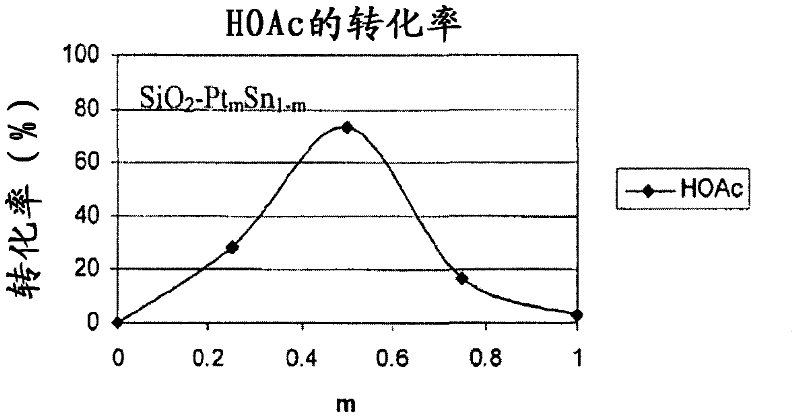
Catalysts for making ethanol from acetic acid
A catalyst and ethanol technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, heterogeneous catalyst chemical elements, etc., can solve the problem that the catalyst does not have the necessary selectivity, the catalyst life is insufficient, and the ethanol production is non-selective, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1 - SiO 2 -CaSiO 3 (5)-Pt(3)-Sn(1.8) catalyst
[0136] The catalyst was firstly prepared by CaSiO 3 (Aldrich) added to SiO 2 Catalyst support, followed by adding Pt / Sn for preparation. First, CaSiO 3 (≤200 mesh) aqueous suspension by adding 0.52 g of the solid to 13 ml of deionized water, followed by adding 1.0 ml of colloidal SiO 2 (15% by weight solution, MALCO) for preparation. The suspension was stirred at room temperature for 2 h, and then 10.0 g of SiO was added using the incipient wetness impregnation technique 2 Catalyst support (14 / 30 mesh). After standing for 2 hours, the material was evaporated to dryness, then dried overnight at 120° C. under circulating air and calcined at 500° C. for 6 hours. Then all SiO 2 -CaSiO 3 Material for Pt / Sn metal impregnation.
[0137] The catalyst is obtained by first adding Sn(OAc) 2 (tin acetate, Sn(OAc) from Aldrich 2 ) (0.4104 g, 1.73 mmol) was prepared by adding to a vial containing 6.75 ml of 1:1 dilu...
Embodiment 2-KA16
[0138] Example 2-KA160-CaSiO 3 (8)-Pt(3)-Sn(1.8)
[0139] The material is prepared by first adding CaSiO 3 Added to KA160 catalyst carrier (SiO 2 -(0.05)Al 2 o 3 , Sud Chemie, 14 / 30 mesh), followed by the addition of Pt / Sn for preparation. First, CaSiO 3 (≤200 mesh) aqueous suspension by adding 0.42 g of the solid to 3.85 ml of deionized water, followed by adding 0.8 ml of colloidal SiO 2 (15% by weight solution, NALCO) for preparation. The suspension was stirred at room temperature for 2 hours and then 5.0 g of KA160 catalyst support (14 / 30 mesh) were added using the incipient wetness impregnation technique. After standing for 2 hours, the material was evaporated to dryness, then dried overnight at 120° C. under circulating air and calcined at 500° C. for 6 hours. Then all KA160-CaSiO 3 Material for Pt / Sn metal impregnation.
[0140] The catalyst is obtained by first adding Sn(OAc) 2 (tin acetate, Sn(OAc) from Aldrich 2 ) (0.2040 g, 0.86 mmol) was added to a vial ...
Embodiment 3
[0141] Example 3 - SiO 2 -CaSiO 3 (2.5)-Pt(1.5)-Sn(0.9)
[0142] The catalyst was prepared in the same manner as in Example 1 with the following starting material: 0.26 g CaSiO 3 As a carrier modifier; 0.5ml colloidal SiO 2 (15% by weight solution, MALCO), 0.3355g (0.86mmol) of Pt(NH 3 ) 4 (NO 3 ) 2 ; and 0.2052 g (0.86 mmol) of Sn(OAc) 2 . Yield: 10.90 g dark gray material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com