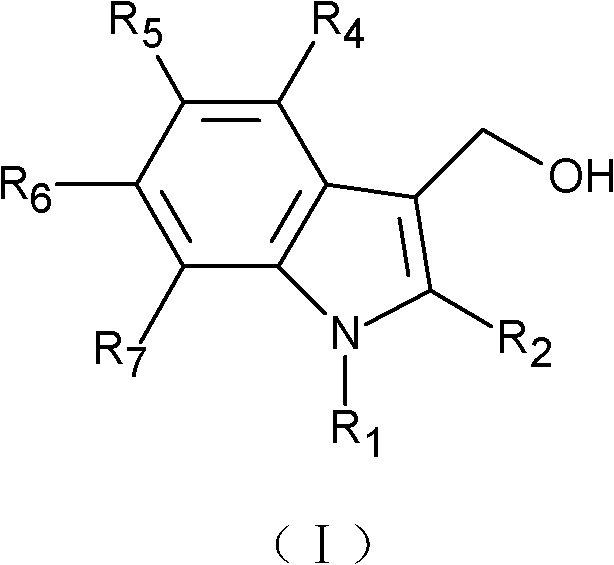
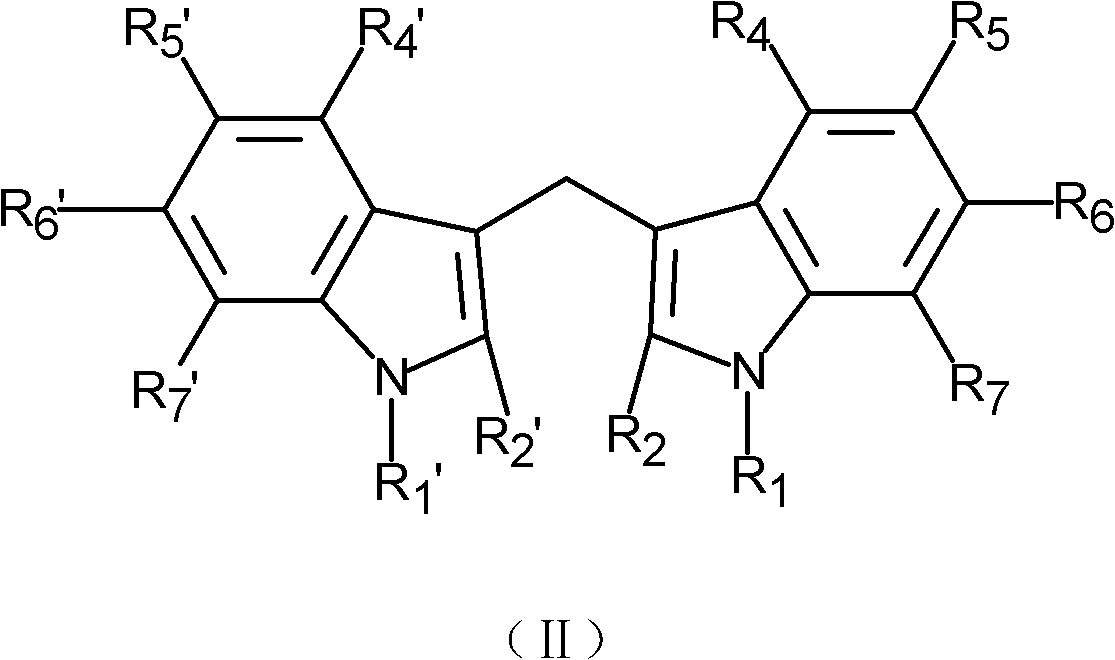
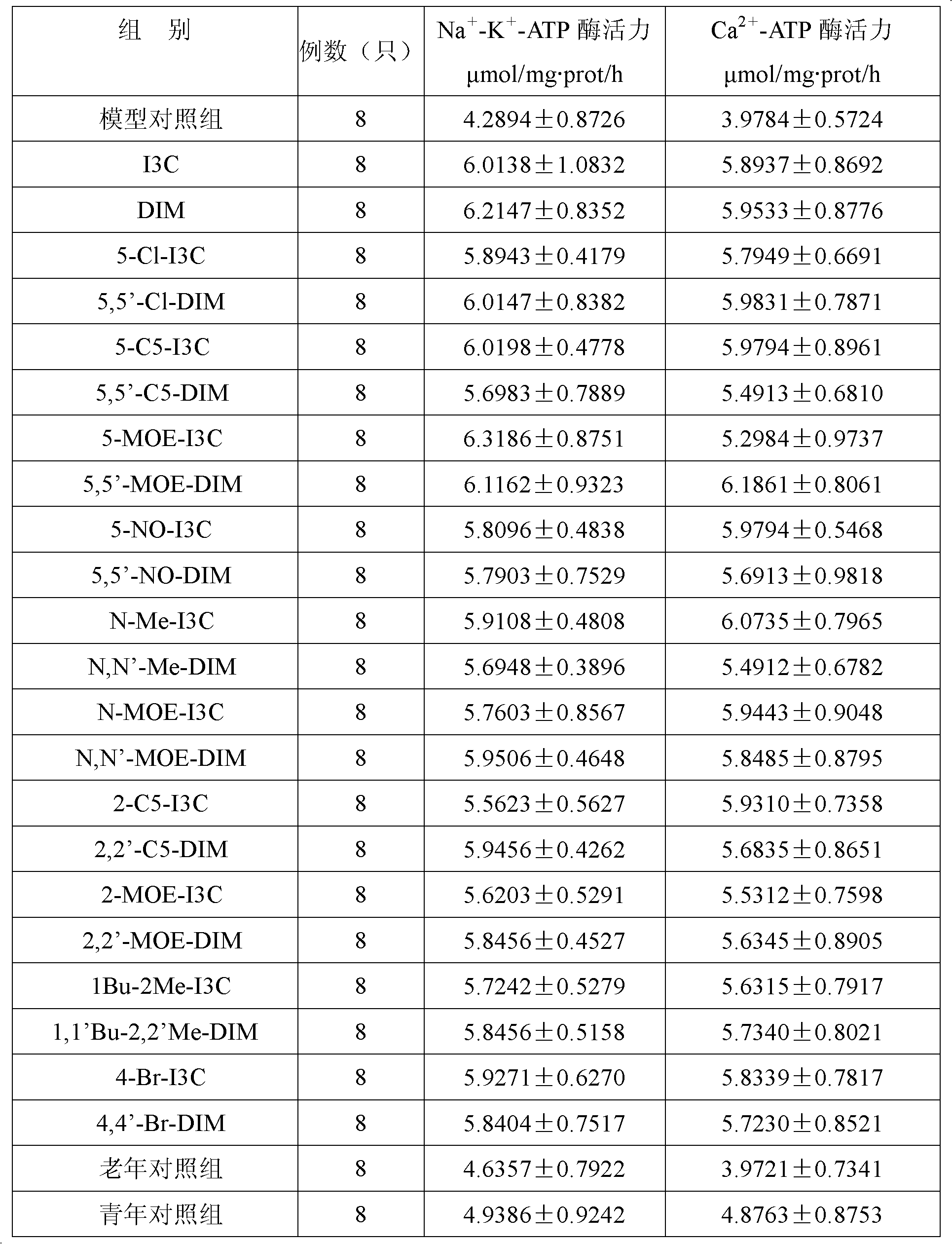
Application of indole-3-carbinol, diindolyl methane and derivatives thereof in preparation of medicaments for treating senile dementia
A diindolylmethane and senile dementia technology, applied in the field of biomedicine, can solve problems such as insufficient effectiveness and specificity, difficulty in penetrating the blood-brain barrier, and clinical drug restrictions, and achieve low price, improvement of movement disorders, and easy storage and transport effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (Preparation of 5-chloroindole-3-methanol and 5,5'-dichlorodiindolylmethane)
[0028] Slowly add 0.86ml of phosphorus oxychloride to 2.9ml of dimethylformamide that has been cooled to 0°C in advance. 8.6mmol 5-chloroindole (purchased from Nanjing Ruima Fine Chemical Co., Ltd.) was dissolved in 1.0ml of dimethylformamide, and then slowly added to the aforementioned precooled phosphorus oxychloride solution, the formed suspension was at 37 °C for 60 minutes until the clear yellow solution turns into a pale yellow paste. Then 1 ml of ice water was added to the pasty mass, followed by slow addition of 10 ml of an aqueous solution containing 3.75 g of KOH. Heat this mixture to boil and then cool, filter, wash with water, and dry in air to obtain 5-chloroindole-3-acetaldehyde.
[0029] 1.0 g of 5-chloroindole-3-acetaldehyde was dissolved in 5.0 ml of methanol, and solid sodium borohydride was continuously added until excess. Then 50ml of water was added to the reactant, co...
Embodiment 2
[0032] (Preparation of 5-nitroindole-3-methanol and 5,5'-dinitrodiindolylmethane)
[0033] 5-Nitroindole can be purchased commercially (Nanjing Ruima Fine Chemical Co., Ltd.). Slowly add 0.92ml of phosphorus oxychloride to 2.9ml of dimethylformamide cooled to 0°C in advance. Dissolve 8.2mmol of 5-nitroindole in 1.0ml of dimethylformamide, then slowly add it to the aforementioned pre-cooled phosphorus oxychloride solution, and heat the formed suspension at 42°C for 90 minutes until a clear yellow solution Turned into a light yellow pasty substance. Then 1 ml of ice water was added to the pasty mass, followed by slow addition of 10 ml of an aqueous solution containing 3.75 g of KOH. Heat this mixture to boil and then cool, filter, wash with water, and dry in air to obtain 5-nitroindole-3-acetaldehyde.
[0034] 1.0 g of 5-nitroindole-3-acetaldehyde was dissolved in 5.0 ml of methanol, and solid sodium borohydride was continuously added until excess. Then 50ml of water was add...
Embodiment 3
[0037] (Preparation of 5-pentylindole-3-carbinol and 5,5'-dipentyl-diindolylmethane)
[0038] 5-Pentylindole can be purchased commercially (Nanjing Ruima Fine Chemical Co., Ltd.). Slowly add 0.82 ml of phosphorus oxychloride to 2.9 ml of dimethylformamide cooled to 0°C in advance. Dissolve 9.2mmol of 5-pentylindole in 1.0ml of dimethylformamide, then slowly add to the aforementioned pre-cooled phosphorus oxychloride solution, and heat the formed suspension at 37°C for 40-60 minutes until clear The yellow solution turned into a pale yellow paste. Then 1 ml of ice water was added to the pasty mass, followed by slow addition of 10 ml of an aqueous solution containing 3.75 g of KOH. Heat this mixture to boil and then cool, filter, wash with water, and dry in air to obtain 5-pentylindole-3-acetaldehyde.
[0039] 1.0 g of 5-pentylindole-3-acetaldehyde was dissolved in 5.0 ml of methanol, and solid sodium borohydride was continuously added until excess. Then 50ml of water was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com