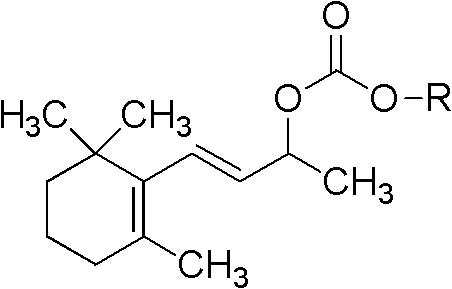
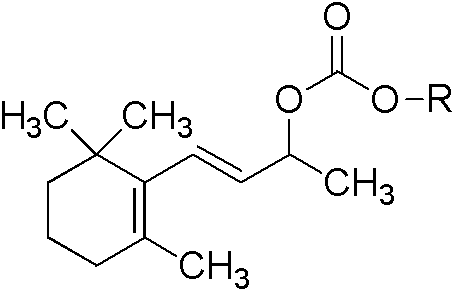
Monosaccharide beta-ionol carbonic acid monoester compound, and preparation method and purpose
The technology of ionol ester and ionol is applied in the fields of monosaccharide β-ionol carbonate monoester compound, new cigarette moisturizing agent, and monosaccharide ester cigarette moisturizing agent, which can solve the problem of uneven fragrance release, loss of fragrance, Problems such as low molecular weight, to achieve the effect of comfortable and harmonious aroma, slowing changes and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
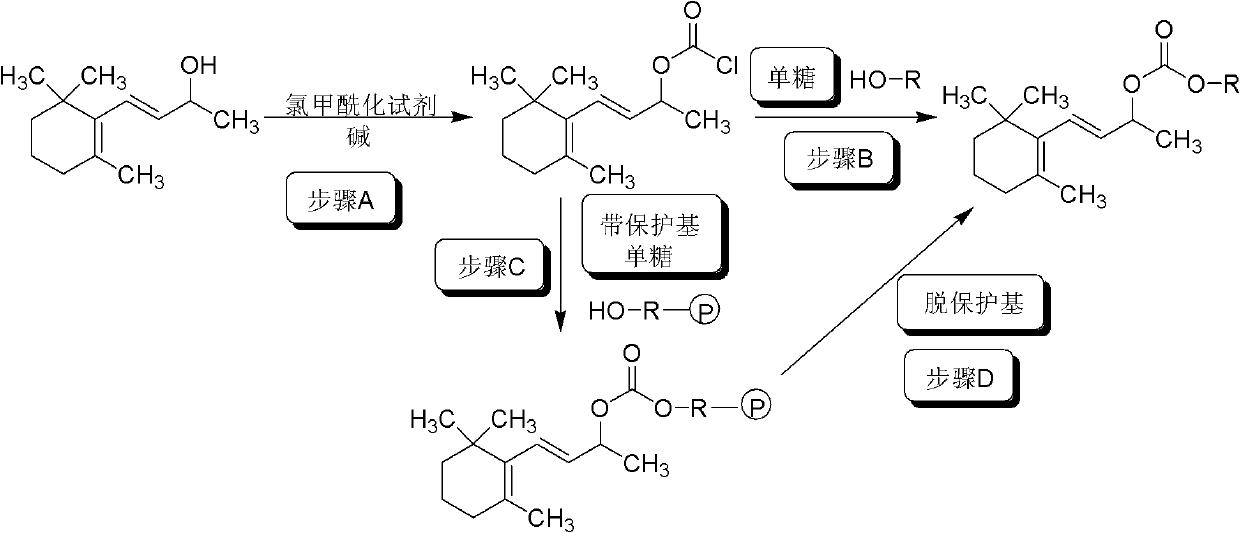
[0042] Embodiment 1: Synthesis of chloroformic acid-β-ionyl ester
[0043] Add 2000ml CH to the reaction flask 2 Cl 2 and bis(trichloromethyl)carbonate 129g (0.44mol), stirred at room temperature until the solid was completely dissolved, put the reaction bottle in an ice-salt bath, cooled to -10°C, added β-ionol 1.28mol, completely dissolved Finally, add 200ml of pyridine dropwise, control the drop rate, and keep the temperature of the reaction solution at 0-5°C. After dropping, the reaction solution is naturally warmed to room temperature, and the reaction is stirred overnight to obtain the dichloroformic acid-β-ionyl dichloride The methane solution can be used in the next step reaction without purification, and the yield is quantitative.
Embodiment 2
[0044] Embodiment 2: Synthesis of chloroformic acid-β-ionyl ester
[0045] The operation process is the same as in Example 1, except that bis(trichloromethyl) carbonate is replaced with trichloromethyl chloroformate, pyridine is replaced with sodium bicarbonate, CH 2 Cl 2 Substitute toluene to obtain a toluene solution of chloroformic acid-β-ionyl ester, which can be used in the next reaction without purification, and the yield is quantitative.
Embodiment 3
[0046] Embodiment 3: Preparation of 3-O-β-ionol carbonyl-D-glucopyranosyl ester (compound I)
[0047]
[0048] Take 0.06 mol of the chloroformic acid-β-ionol ester dichloromethane solution prepared in Example 1, and add dropwise to 1,2:5,6-di-O-isopropylidene- D-glucofuranose 0.05mol, triethylamine 0.08mol and dichloromethane 100ml mixed solution, stirred at room temperature for 20h, after the reaction, the reaction solution was transferred to a separatory funnel, and the organic layer was sequentially washed with 5% HCl aqueous solution 25ml , saturated NaHCO 3 25ml of aqueous solution and 25ml of saturated NaCl aqueous solution, washed with anhydrous NaCl 2 SO 4 Dry, filter, evaporate the solvent under reduced pressure, add 50ml of 70% trifluoroacetic acid aqueous solution to the residue, stir and react at 15-25°C for 3h, evaporate the solvent under reduced pressure, and obtain 3-O-β-ionol carbonyl-D-glucose The crude pyranose ester was purified by silica gel column ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com